
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
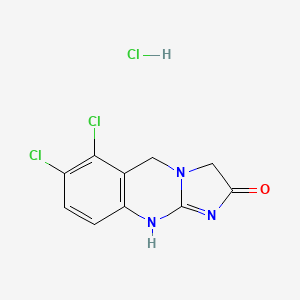

| Molecular Weight | 292.5 g/mol |
|---|---|
| Molecular Formula | C10H8Cl3N3O |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 290.973295 g/mol |
| Monoisotopic Mass | 290.973295 g/mol |
| Topological Polar Surface Area | 44.7 A^2 |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 360 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Agrylin |
| PubMed Health | Anagrelide (By mouth) |
| Drug Classes | Platelet Reducing Agent |
| Drug Label | Name: AGRYLIN (anagrelide hydrochloride)Dosage Form:0.5 mg capsules for oral administrationActive Ingredient:AGRYLIN Capsules contain 0.5 mg of anagrelide base (as anagrelide hydrochloride).Inactive Ingredients:Anhydrous Lactose NF, Crospov... |
| Active Ingredient | Anagrelide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 0.5mg base |
| Market Status | Prescription |
| Company | Shire |
| 2 of 4 | |
|---|---|
| Drug Name | Anagrelide hydrochloride |
| Drug Label | Anagrelide hydrochloride is an off white powder that is very slightly soluble in water and sparingly soluble in dimethyl sulfoxide and in dimethylformamide. Anagrelide hydrochloride is a platelet-reducing agent with a chemical name of 6,7-dichloro-1,... |
| Active Ingredient | Anagrelide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 0.5mg base; eq 1mg base |
| Market Status | Prescription |
| Company | Mylan Pharms; Ivax Sub Teva Pharms; Mylan; Impax Labs; Barr |
| 3 of 4 | |
|---|---|
| Drug Name | Agrylin |
| PubMed Health | Anagrelide (By mouth) |
| Drug Classes | Platelet Reducing Agent |
| Drug Label | Name: AGRYLIN (anagrelide hydrochloride)Dosage Form:0.5 mg capsules for oral administrationActive Ingredient:AGRYLIN Capsules contain 0.5 mg of anagrelide base (as anagrelide hydrochloride).Inactive Ingredients:Anhydrous Lactose NF, Crospov... |
| Active Ingredient | Anagrelide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 0.5mg base |
| Market Status | Prescription |
| Company | Shire |
| 4 of 4 | |
|---|---|
| Drug Name | Anagrelide hydrochloride |
| Drug Label | Anagrelide hydrochloride is an off white powder that is very slightly soluble in water and sparingly soluble in dimethyl sulfoxide and in dimethylformamide. Anagrelide hydrochloride is a platelet-reducing agent with a chemical name of 6,7-dichloro-1,... |
| Active Ingredient | Anagrelide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 0.5mg base; eq 1mg base |
| Market Status | Prescription |
| Company | Mylan Pharms; Ivax Sub Teva Pharms; Mylan; Impax Labs; Barr |
Date of Issue : 2022-06-20
Valid Till : 2025-08-08
Written Confirmation Number : WC-0113
Address of the Firm : Old Madras Road, Virgo Nagar Post, Bengaluru - 560049

Date of Issue : 2020-02-14
Valid Till : 2022-07-02
Written Confirmation Number : WC-0214
Address of the Firm : Gut No. 546, 571, 519 & 520, Village: Kumbhavali, Tarapur, Boisar, Tal & DIst- P...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?