
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
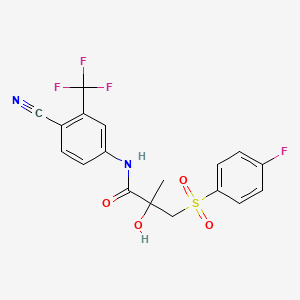

1. 4'-cyano-3-(4-fluorophenylsulfonyl)-2-hydroxy-2-methyl-3'-(trifluoromethyl)propionanilide
2. Casodex
3. Cosudex
4. Ici 176334
5. Ici-176334
1. 90357-06-5
2. Casodex
3. Bicalutamide (cdx)
4. Calutide
5. Ici 176334
6. Ici-176334
7. N-(4-cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methylpropanamide
8. N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenyl)sulfonyl-2-hydroxy-2-methylpropanamide
9. Ici 176,334
10. Bicalutamide (casodex)
11. Cosudex
12. A0z3nau9dp
13. Chembl409
14. Nsc-759816
15. N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide
16. Ici176,334-1
17. Propanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-
18. Raffolutil
19. Kalumid
20. Smr000466329
21. Casodex (tn)
22. Sr-01000759410
23. Unii-a0z3nau9dp
24. Brn 5364666
25. Bicalutamine
26. Bicalutamide (jan/usp/inn)
27. Propanamide,
28. Ccris 8728
29. Hsdb 7655
30. Bicalutamide [usan:usp:inn:ban]
31. Mfcd00869971
32. (+-)-4'-cyano-alpha,alpha,alpha-trifluoro-3-((p-fluorophenyl)sulfonyl)-2-methyl-m-lactotoluidide
33. Ks-1161
34. Bicalutamide - Casodex
35. Cpd000466329
36. Bicalutamide [mi]
37. Bicalutamide [inn]
38. Bicalutamide [jan]
39. Bicalutamide [hsdb]
40. Bicalutamide [usan]
41. Schembl3611
42. Bicalutamide [vandf]
43. Bicalutamide [mart.]
44. (r)-(-)-bicalutamide-d4
45. Mls000759437
46. Mls001424047
47. Propanamide, N-(4-cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methyl-, (+-)-
48. S-(+)-bicalutamide-[d4]
49. Bicalutamide [usp-rs]
50. Bicalutamide [who-dd]
51. Gtpl2863
52. Dtxsid2022678
53. Bdbm18525
54. Chebi:91617
55. Ex-a962
56. Chebi:144093
57. Bcpp000337
58. Bicalutamide [orange Book]
59. Hms2051b13
60. Hms2089n12
61. Hms2232h03
62. Hms3263m13
63. Hms3372k05
64. Hms3393b13
65. Hms3654k18
66. Hms3714p13
67. Pharmakon1600-01504827
68. Bicalutamide [ep Monograph]
69. Bicalutamide [usp Impurity]
70. Act06291
71. Amy33430
72. Bcp02110
73. Bicalutamide [usp Monograph]
74. Tox21_501026
75. Nsc722665
76. Nsc759816
77. S1190
78. Akos015895073
79. Ac-4232
80. Bcp9000408
81. Ccg-100951
82. Ccg-220876
83. Ccg-222330
84. Cs-1296
85. Db01128
86. Lp01026
87. Nc00201
88. Nsc 759816
89. Nsc-722665
90. Sb17301
91. Sdccgsbi-0633779.p001
92. N-(4-cyano-3-(trifluoromethyl)phenyl)
93. Ncgc00167977-01
94. Ncgc00167977-02
95. Ncgc00167977-03
96. Ncgc00167977-09
97. Ncgc00167977-20
98. Ncgc00261711-01
99. Hy-14249
100. Db-041165
101. B3206
102. Ft-0618286
103. Ft-0631069
104. Ft-0663100
105. Sw197581-4
106. Bicalutamide (cdx), >=98% (hplc), Powder
107. C08160
108. D00961
109. Ab00639963-06
110. Ab00639963-08
111. Ab00639963-09
112. Ab00639963_10
113. 357b065
114. A803039
115. A843528
116. Q1988832
117. Sr-01000759410-4
118. Sr-01000759410-5
119. Brd-a29485665-001-03-7
120. Bicalutamide, British Pharmacopoeia (bp) Reference Standard
121. Bicalutamide, European Pharmacopoeia (ep) Reference Standard
122. Bicalutamide, United States Pharmacopeia (usp) Reference Standard
123. 4'-cyano-3-[(4- Fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-3'-trifluoromethylpropionanilide
124. 4'-cyano-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-3'-trifluoromethylpropionanilide
125. Bicalutamide For System Suitability, European Pharmacopoeia (ep) Reference Standard
126. Bicalutamide, Pharmaceutical Secondary Standard; Certified Reference Material
127. (+/-)-4'-cyano-.alpha.,.alpha.,.alpha.-trifluoro-3-((p-fluorophenyl)sulfonyl)-2-methyl-m-lactotoluidide
128. N-(4-cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methyl-n-phenylpropanamide
129. N-(4-cyano-3-(trifluoromethyl)phenyl)-3-(4-fluorophenylsulfonyl)-2-hydroxy-2-methylpropanamide
130. N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenyl)sulfonyl-2-methyl-2-oxidanyl-propanamide
131. N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorobenzene)sulfonyl]-2-hydroxy-2-methylpropanamide
132. N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropionamide
133. N-[4-cyano-3-trifluoromethyl-phenyl]-3-[4-fluorophenyl-sulfonyl]-2-hydroxy-2-methyl-propionamide
134. Propanamide, N-(4-cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methyl-, (+/-)-
| Molecular Weight | 430.4 g/mol |
|---|---|
| Molecular Formula | C18H14F4N2O4S |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 430.06104075 g/mol |
| Monoisotopic Mass | 430.06104075 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 750 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Bicalutamide |
| PubMed Health | Bicalutamide (By mouth) |
| Drug Classes | Antiandrogen |
| Drug Label | Bicalutamide tablets contain 50 mg of bicalutamide, a non-steroidal androgen receptor inhibitor with no other known endocrine activity. The chemical name is propanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-m... |
| Active Ingredient | Bicalutamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Actavis Elizabeth; Fresenius Kabi Oncol; Teva; Accord Hlthcare; Zydus Pharms Usa; Sandoz; Mylan; Sun Pharma Global |
| 2 of 4 | |
|---|---|
| Drug Name | Casodex |
| PubMed Health | Bicalutamide (By mouth) |
| Drug Classes | Antiandrogen |
| Drug Label | CASODEX (bicalutamide) Tablets contain 50 mg of bicalutamide, a non-steroidal androgen receptor inhibitor with no other known endocrine activity. The chemical name is propanamide, N [4 cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2... |
| Active Ingredient | Bicalutamide |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Astrazeneca |
| 3 of 4 | |
|---|---|
| Drug Name | Bicalutamide |
| PubMed Health | Bicalutamide (By mouth) |
| Drug Classes | Antiandrogen |
| Drug Label | Bicalutamide tablets contain 50 mg of bicalutamide, a non-steroidal androgen receptor inhibitor with no other known endocrine activity. The chemical name is propanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-m... |
| Active Ingredient | Bicalutamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Actavis Elizabeth; Fresenius Kabi Oncol; Teva; Accord Hlthcare; Zydus Pharms Usa; Sandoz; Mylan; Sun Pharma Global |
| 4 of 4 | |
|---|---|
| Drug Name | Casodex |
| PubMed Health | Bicalutamide (By mouth) |
| Drug Classes | Antiandrogen |
| Drug Label | CASODEX (bicalutamide) Tablets contain 50 mg of bicalutamide, a non-steroidal androgen receptor inhibitor with no other known endocrine activity. The chemical name is propanamide, N [4 cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2... |
| Active Ingredient | Bicalutamide |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Astrazeneca |
Androgen Antagonists; Antineoplastic Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/Bicalutamide/ 50 mg daily is indicated for use in combination therapy with a luteinizing hormone-releasing hormone (LHRH) analogue for the treatment of Stage D2 metastatic carcinoma of the prostate. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Casodex (Bicalutamide) (August 2007). Available from, as of November 12, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4740
/Bicalutamide/ 150 mg daily is not approved for use alone or with other treatments. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Casodex (Bicalutamide) (August 2007). Available from, as of November 12, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4740
/Bicalutamide/ is contraindicated in any patient who has shown a hypersensitivity reaction to the drug or any of the tablet's components.
US Natl Inst Health; DailyMed. Current Medication Information for Casodex (Bicalutamide) (August 2007). Available from, as of November 12, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4740
/Bicalutamide/ has no indication for women, and should not be used in this population, particularly for non-serious or non-life threatening conditions.
US Natl Inst Health; DailyMed. Current Medication Information for Casodex (Bicalutamide) (August 2007). Available from, as of November 12, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4740
FDA Pregnancy Risk Category: X /CONTRAINDICATED IN PREGNANCY. Studies in animals and or humans, or investigational or post-marketing reports, have demonstrated positive evidence of fetal abnormalities or risk which clearly outweighs any possible benefit to the patient./
US Natl Inst Health; DailyMed. Current Medication Information for Casodex (Bicalutamide) (August 2007). Available from, as of November 12, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4740
It is not known whether /bicalutamide/ drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when /bicalutamide/ is administered to a nursing woman.
US Natl Inst Health; DailyMed. Current Medication Information for Casodex (Bicalutamide) (August 2007). Available from, as of November 12, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4740
For more Drug Warnings (Complete) data for BICALUTAMIDE (10 total), please visit the HSDB record page.
For treatment (together with surgery or LHRH analogue) of advanced prostatic cancer.
FDA Label
Bicalutamide is an antineoplastic hormonal agent primarily used in the treatment of prostate cancer. Bicalutamide is a pure, nonsteroidal anti-androgen with affinity for androgen receptors (but not for progestogen, estrogen, or glucocorticoid receptors). Consequently, Bicalutamide blocks the action of androgens of adrenal and testicular origin which stimulate the growth of normal and malignant prostatic tissue. Prostate cancer is mostly androgen-dependent and can be treated with surgical or chemical castration. To date, antiandrogen monotherapy has not consistently been shown to be equivalent to castration.
Androgen Antagonists
Compounds which inhibit or antagonize the biosynthesis or actions of androgens. (See all compounds classified as Androgen Antagonists.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
L02BB03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02B - Hormone antagonists and related agents
L02BB - Anti-androgens
L02BB03 - Bicalutamide
Absorption
Bicalutamide is well-absorbed following oral administration, although the absolute bioavailability is unknown.
Clearance
Apparent oral cl=0.32 L/h [Normal Males]
Bicalutamide is well-absorbed following oral administration, although the absolute bioavailability is unknown. Co-administration of bicalutamide with food has no clinically significant effect on rate or extent of absorption.
US Natl Inst Health; DailyMed. Current Medication Information for Casodex (Bicalutamide) (August 2007). Available from, as of November 12, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4740
Bicalutamide is highly protein-bound (96%).
US Natl Inst Health; DailyMed. Current Medication Information for Casodex (Bicalutamide) (August 2007). Available from, as of November 12, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4740
... Bicalutamide metabolites are excreted almost equally in urine and feces with little or no unchanged drug excreted in urine; conversely, unchanged drug predominates in plasma. Bicalutamide in feces is thought to arise from hydrolysis of bicalutamide glucuronide and from unabsorbed drug. ...
PMID:15509184 Cockshott ID; Clin Pharmacokinet 43 (13): 855-78 (2004)
... Healthy male volunteers (n = 15) were administered single oral doses of bicalutamide (50 mg) after food and after fasting as part of a three-treatment, three-period, randomized cross-over study, with a 9 week washout. After fasting, plasma concentrations of (R)-bicalutamide were much higher than those of (S)-bicalutamide; the mean (R)-enantiomer Cmax (734 ng mL-1) was about nine times higher than the (S)-enantiomer value (84 ng mL-1). The corresponding tmax values were 19 and 3 hr for (R)- and (S)-bicalutamide, respectively. Elimination of (R)-bicalutamide from plasma was monoexponential and slow (t1/2 = 5.8 d). Elimination of (S)-bicalutamide was biphasic in some volunteers but monophasic in others (terminal t1/2 =1.2 d; n = 11). There was no significant effect of food on AUC, tmax, or t1/2 data for either enantiomer. The observed slightly higher values of Cmax for (R)-bicalutamide (14%) and (S)-bicalutamide (19%), when dosing with food, achieved statistical significance. ...
PMID:9312310 Cockshott ID et al; Biopharm Drug Dispos 18 (6): 499-507 (1997)
For more Absorption, Distribution and Excretion (Complete) data for BICALUTAMIDE (9 total), please visit the HSDB record page.
Bicalutamide undergoes stereo specific metabolism. The S (inactive) isomer is metabolized primarily by glucuronidation. The R (active) isomer also undergoes glucuronidation but is predominantly oxidized to an inactive metabolite followed by glucuronidation.
Bicalutamide undergoes stereospecific metabolism. The S (inactive) isomer is metabolized primarily by glucuronidation. The R (active) isomer also undergoes glucuronidation but is predominantly oxidized to an inactive metabolite followed by glucuronidation. Both the parent and metabolite glucuronides are eliminated in the urine and feces. The S-enantiomer is rapidly cleared relative to the R-enantiomer, with the R-enantiomer accounting for about 99% of total steady-state plasma levels.
US Natl Inst Health; DailyMed. Current Medication Information for Casodex (Bicalutamide) (August 2007). Available from, as of November 12, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4740
5.9 days
... Elimination of (S)-bicalutamide was biphasic in some volunteers but monophasic in others (terminal t1/2 =1.2 d; n = 11). ...
PMID:9312310 Cockshott ID et al; Biopharm Drug Dispos 18 (6): 499-507 (1997)
/The/ apparent plasma elimination half-life observed following repeated administration was 8.4 +/- 1.1 days.
PMID:8712091 Kotake T et al; Hinyokika Kiyo 42 (2): 143-53 (1996)
Bicalutamide competes with androgen for the binding of androgen receptors, consequently blocking the action of androgens of adrenal and testicular origin which stimulate the growth of normal and malignant prostatic tissue.
Bicalutamide is a nonsteroidal antiandrogen that is structurally and pharmacologically related to flutamide and nilutamide. Bicalutamide inhibits the action of androgens by competitively blocking nuclear androgen receptors in target tissues such as the prostate, seminal vesicles, and adrenal cortex; blockade of androgen receptors in the hormone-sensitive tumor cells may result in growth arrest or transient tumor regression through inhibition of androgen-dependent DNA and protein synthesis. Bicalutamide is a selective antiandrogen with no androgenic or progestational activity in various animal models. The relative binding affinity of bicalutamide at the androgen receptor is more than that of nilutamide and approximately 4 times that of hydroxyflutamide, the active metabolite of flutamide.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 944
Common pharmacologic therapies for prostate cancer (ie, gonadotropin-releasing hormone [GnRH] analogs, nonsteroidal antiandrogens) when used as monotherapy initially result in increased serum testosterone concentrations, which may limit the effects of the drugs. Androgen receptors in the hypothalamus are blocked by bicalutamide, which disrupts the inhibitory feedback of testosterone on luteinizing hormone (LH) release, resulting in a temporary increase in secretion of LH; the increase in LH stimulates an increase in the production of testosterone. As GnRH analogs have potent GnRH agonist properties, testicular steroidogenesis continues during the first few weeks after initiating therapy. However, the combination of orchiectomy or GnRH analog therapy to suppress testicular androgen production and an antiandrogen to block response of remaining adrenal androgens provides maximal androgen blockade. Concomitant administration of antiandrogens such as bicalutamide in patients initiating therapy with a GnRH analog can inhibit initial androgenic stimulation and potential exacerbation of symptoms (e.g., bone pain, urinary obstruction, liver pain, impending spinal cord compression) that may occur during the first month of GnRH analog therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 944
Bicalutamide was developed from a series of nonsteroidal compounds related to flutamide that showed a range of pharmacologic activity from full androgen agonist to pure antiandrogen, including progestational and antiprogestational properties. Bicalutamide is a pure antiandrogen that binds to rat, dog, and human prostate; the affinity compared with the natural ligand 5 alpha-dihydrotestosterone is low, but bicalutamide has an affinity for the rat androgen receptor approximately four times higher than hydroxyflutamide, the active metabolite of flutamide. Bicalutamide also binds to androgen receptors found in the LNCaP human prostate tumor and the Shionogi S115 mouse mammary tumor cell line, as well as androgen receptors transfected into CV-1 and HeLa cells. In all cases, bicalutamide behaves as a pure antiandrogen and inhibits gene expression and cell growth stimulated by androgen. Studies with the LNCaP cell line are particularly interesting, as these cells contain a mutated androgen receptor (codon 868, Thr-->Ala), which behaves idiosyncratically with other antiandrogens (cyproterone acetate and flutamide): both these antiandrogens act as agonists in this cell line and stimulate proliferation. Studies in vivo show that bicalutamide is a potent antiandrogen in the rat. In immature, castrated male rats treated daily with testosterone propionate, bicalutamide produces a profound inhibition of accessory sex organ (ventral prostate and seminal vesicles) growth at oral doses as low as 0.25 mg/kg; it is more active in this test than flutamide or cyproterone acetate. In mature male rats, daily oral doses of bicalutamide produce a dose-related reduction in weights of the ventral prostate glands and seminal vesicles: in this test, bicalutamide is around five times as potent as flutamide. In contrast to flutamide, which produces dose-related, marked increases in serum luteinizing hormone (LH) and testosterone as a consequence of the central inhibition of the negative feedback effects of androgens on the hypothalamic-pituitary-tests axis, bicalutamide has little effect on serum LH and testosterone; i.e., it is peripherally selective. The peripheral selectivity of bicalutamide in the rat is not due to differences between the prostate versus hypothalamic or pituitary receptors, as bicalutamide reverses the suppressive effect of testosterone on luteinizing hormone-releasing hormone (LHRH) secretion from hypothalamic slices in vitro and is as effective as flutamide at sensitizing the pituitary gland to secrete LH in response to administered LHRH. The peripheral selectivity of bicalutamide has now been shown to be due to poor penetration across the blood-brain barrier: tissue distribution studies with [3H]bicalutamide show that although it is concentrated in the organs of metabolism and secretion as well as in the prostate, the pituitary glands, and the seminal vesicles, levels in the hypothalamus and the central nervous system (CNS) are much lower than in blood. Indeed, it is probable that levels found in the CNS reflect levels of blood contamination. In dogs, bicalutamide has exquisite potency and causes dose-related atrophy of the prostate gland and epididymides; with an oral ED50 of 0.1 mg/kg, it is around 50 times as potent as flutamide in this species and also more potent than the steroidal antiandrogen WIN49596 and the 5 alpha-reductase inhibitor MK-906. Even at substantial multiples of the active dose (up to 100 mg/kg orally), bicalutamide failed to increase serum testosterone, so it is also peripherally selective in the dog.
PMID:8560673 Furr B, Tucker H; Urology 47(1A Suppl):13-25; discussion 29-32 (1996)
Although widely accepted as an androgen receptor antagonist, the mechanism by which it induces apoptosis remains unclear. Defining exact pathways by which bicalutamide induces its apoptotic effects would help to advance its clinical applications. /Investigators/ aimed to examine the apoptotic effects of bicalutamide at 24 hr and comment on the role of the caspases and calpains in mediating bicalutamide-induced apoptosis in androgen-dependent and androgen-independent cells. PWR-1E, PC-3 and DU-145 cells were treated with bicalutamide and assessed for apoptosis by flow cytometry at 24 hr. DU-145 cells were used to compare differences between two different metastatic receptor-negative cells and to verify apoptotic induction at 48 hr. To delineate a specific pathway of action for bicalutamide, PC-3 and PWR-1E cells were pretreated with specific inhibitors of caspase-dependent (zVAD-FMK) and caspase-independent pathways (calpain 2 inhibitor). Bicalutamide induced apoptosis in androgen-dependent PWR-1E cells via a caspase-dependent and calpain-independent mechanism. In androgen-independent PC-3 cells, bicalutamide also induced apoptosis by mechanisms that were partially inhibited by pan-caspase inhibition but were partially calpain dependent. Understanding into how bicalutamide exerts its effects in androgen-independent cells will yield further insights into the treatment of hormone-refractory disease.
PMID:18475288 Floyd MS Jr et al; Prostate Cancer Prostatic Dis 12 (1): 25-33 (2008)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?