
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
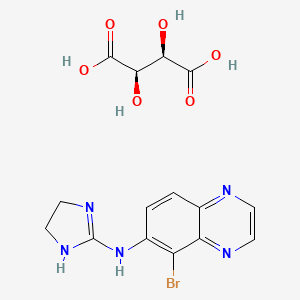

1. 5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline D-tartrate
2. 5-bromo-6-(imidazolidinylideneamino)quinoxaline
3. 5-bromo-6-(imidazolin-2-ylamino)quinoxaline
4. Agn 190342
5. Agn-190342
6. Agn190342
7. Alphagan
8. Alphagan P
9. Brimonidine
10. Brimonidine Purite
11. Brimonidine Tartrate (1:1)
12. Brimonidine Tartrate (1:1), (s-(r*,r*))-isomer
13. Brimonidine Tartrate, (r-(r*,r*))-isomer
14. Bromoxidine
15. Mirvaso
16. Ratio Brimonidine
17. Ratio-brimonidine
18. Sanrosa
19. Uk 14,304
20. Uk 14,304 18
21. Uk 14,304-18
22. Uk 14,30418
23. Uk 14,308
24. Uk 14304
25. Uk 14308
26. Uk-14,304-18
27. Uk-14,308
28. Uk-14304
29. Uk14,30418
30. Uk14,308
31. Uk14304
1. 70359-46-5
2. Alphagan
3. Brimonidine Tartarate
4. Brimonidine L-tartrate
5. Lumify
6. Brimonidinne Tartrate
7. 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)quinoxalin-6-amine (2r,3r)-2,3-dihydroxysuccinate
8. Mirvaso
9. 4s9cl2dy2h
10. Agn 190342-lf
11. Brimonidine D-tartarate
12. 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)quinoxalin-6-amine;(2r,3r)-2,3-dihydroxybutanedioic Acid
13. Uk-14304-18
14. Qoliana
15. Agn-190342-lf
16. Bromoxidine Tartrate
17. (5-bromo-quinoxalin-6-yl)-(4,5-dihydro-1h-imidazol-2-yl)-amine L-tartrate
18. Brimonidine Purite
19. Brimonidine Tartrate [usan]
20. (2r,3r)-2,3-dihydroxybutanedioic Acid; 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)quinoxalin-6-amine
21. 6-quinoxalinamine, 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)-, (2r,3r)-2,3-dihydroxybutanedioate (1:1)
22. 6-quinoxalinamine, 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)-, (r-(r*,r*))-2,3-dihydroxybutanedioate (1:1)
23. 59803-99-5
24. Ocu300
25. Unii-4s9cl2dy2h
26. Ocu-300
27. Agn-190342lf
28. Alphagan-p
29. Cd-07805
30. Agn 190342lf
31. Brimonidine Tartrate [usan:jan]
32. N-(5-bromoquinoxalin-6-yl)imidazolidin-2-imine;(2r,3r)-2,3-dihydroxybutanedioic Acid
33. Alphagan (tn)
34. 304 Tartrate
35. Uk-1430418
36. 5-bromo-n-
37. Uk14304 Tartrate
38. Agn190342 Tartrate
39. Uk 14304 (tartrate)
40. Agn190342 (tartrate)
41. Uk 14,304 (tartrate)
42. Schembl265607
43. Chembl1200389
44. Ex-a5415a
45. Hy-b0659a
46. Dtxsid70911371
47. Brimonidine Tartrate (jan/usan)
48. Brimonidine Tartrate [jan]
49. Hms3715p18
50. Act08633
51. Brimonidine D-tartrate [mi]
52. Brimonidine Tartrate [vandf]
53. Brimonidine Tartrate [mart.]
54. Mfcd07773072
55. Brimonidine Tartrate [usp-rs]
56. Brimonidine Tartrate [who-dd]
57. Akos016845265
58. Ccg-221278
59. Cs-4496
60. 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)quinoxalin-6-amine(2r,3r)-2,3-dihydroxysuccinate
61. As-18083
62. Brimonidine Tartrate [orange Book]
63. Brimonidine Tartrate [ep Monograph]
64. Brimonidine Tartrate [usp Monograph]
65. Cd07805/47
66. Cd-07805/47
67. Combigan Component Brimonidine Tartrate
68. A19740
69. D02076
70. Simbrinza Component Brimonidine Tartrate
71. Brimonidine Tartrate Component Of Combigan
72. 359b465
73. Brimonidine Tartrate Component Of Simbrinza
74. (4,5-bihydro-1h-imidazol-2-yl)quinoxalin-6-amine L-tartrate
75. 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl) Quinoxalin-6-amine Tartrate
76. 109826-56-4
| Molecular Weight | 442.22 g/mol |
|---|---|
| Molecular Formula | C15H16BrN5O6 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 441.02840 g/mol |
| Monoisotopic Mass | 441.02840 g/mol |
| Topological Polar Surface Area | 177 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 442 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Brimonidine tartrate |
| Drug Label | Brimonidine Tartrate Ophthalmic Solution, 0.2% is a relatively selective alpha-2 adrenergic agonist for ophthalmic use. The chemical name of brimonidine tartrate is 5-bromo-6 (2-imidazolidinylideneamino) quinoxaline L-tartrate. It is a white to sligh... |
| Active Ingredient | Brimonidine tartrate |
| Dosage Form | Solution/drops; Solution |
| Route | ophthalmic; Ophthalmic |
| Strength | 0.2%; 0.1% |
| Market Status | Tentative Approval; Prescription |
| Company | Apotex; Bausch And Lomb; Alcon Pharms; Sandoz; Akorn |
| 2 of 2 | |
|---|---|
| Drug Name | Brimonidine tartrate |
| Drug Label | Brimonidine Tartrate Ophthalmic Solution, 0.2% is a relatively selective alpha-2 adrenergic agonist for ophthalmic use. The chemical name of brimonidine tartrate is 5-bromo-6 (2-imidazolidinylideneamino) quinoxaline L-tartrate. It is a white to sligh... |
| Active Ingredient | Brimonidine tartrate |
| Dosage Form | Solution/drops; Solution |
| Route | ophthalmic; Ophthalmic |
| Strength | 0.2%; 0.1% |
| Market Status | Tentative Approval; Prescription |
| Company | Apotex; Bausch And Lomb; Alcon Pharms; Sandoz; Akorn |
Mirvaso is indicated for the symptomatic treatment of facial erythema of rosacea in adult patients.
Adrenergic alpha-2 Receptor Agonists
Compounds that bind to and activate ADRENERGIC ALPHA-2 RECEPTORS. (See all compounds classified as Adrenergic alpha-2 Receptor Agonists.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
D11AX21

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AT
Brand Name : ALPHAGAN P
Dosage Form : SOLUTION/DROPS;OPHTHALMIC
Dosage Strength : 0.15%
Approval Date : 2001-03-16
Application Number : 21262
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AT
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
BRIMONIDINE TARTRATE; TIMOLOL MALEATE
Brand Name : COMBIGAN
Dosage Form : SOLUTION/DROPS;OPHTHALMIC
Dosage Strength : 0.2%;EQ 0.5% BASE
Approval Date : 2007-10-30
Application Number : 21398
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
Brand Name : ALPHAGAN P
Dosage Form : SOLUTION/DROPS;OPHTHALMIC
Dosage Strength : 0.1%
Approval Date : 2005-08-19
Application Number : 21770
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ALPHAGAN
Dosage Form : SOLUTION/DROPS;OPHTHALMIC
Dosage Strength : 0.5%
Approval Date : 1997-03-13
Application Number : 20490
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : ALPHAGAN
Dosage Form : SOLUTION/DROPS;OPHTHALMIC
Dosage Strength : 0.2% **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 1996-09-06
Application Number : 20613
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code :
Brand Name : BRIMONIDINE TARTRATE
Dosage Form : SOLUTION/DROPS;OPHTHALMIC
Dosage Strength : 0.025%
Approval Date : 2024-02-16
Application Number : 216361
RX/OTC/DISCN : OTC
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
BRIMONIDINE TARTRATE; TIMOLOL MALEATE
Brand Name : BRIMONIDINE TARTRATE AND TIMOLOL MALEATE
Dosage Form : SOLUTION/DROPS;OPHTHALMIC
Dosage Strength : 0.2%;EQ 0.5% BASE
Approval Date : 2023-08-25
Application Number : 215230
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : BRIMONIDINE TARTRATE
Dosage Form : SOLUTION/DROPS;OPHTHALMIC
Dosage Strength : 0.1%
Approval Date : 2022-12-21
Application Number : 78480
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
BRIMONIDINE TARTRATE; TIMOLOL MALEATE
Brand Name : BRIMONIDINE TARTRATE AND TIMOLOL MALEATE
Dosage Form : SOLUTION/DROPS;OPHTHALMIC
Dosage Strength : 0.2%;EQ 0.5% BASE
Approval Date : 2022-04-04
Application Number : 91087
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
BRIMONIDINE TARTRATE; TIMOLOL MALEATE
Brand Name : BRIMONIDINE TARTRATE AND TIMOLOL MALEATE
Dosage Form : SOLUTION/DROPS;OPHTHALMIC
Dosage Strength : 0.2%;EQ 0.5% BASE
Approval Date : 2022-10-31
Application Number : 91086
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : ALPHAGAN
Dosage Form : Eye Drops Solution
Dosage Strength : 0.20%
Packaging : 5 ML 0.2% - OPHTHALMIC USE SOLUTION
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : COMBIGAN
Dosage Form : Eye Drops
Dosage Strength : 0.2% + 0.5%
Packaging : 5 ML 2 MG/ML + 5 MG/ML - OPHTHALMIC USE
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Alphagan
Dosage Form : Gt Opht
Dosage Strength : 0.10%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Alphagan
Dosage Form : Gt Opht
Dosage Strength : 0.20%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brimonidini tartras; Timololum
Brand Name : Combigan
Dosage Form : Gtt Opht
Dosage Strength :
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brimonidini tartras; Timololum
Brand Name : Combigan
Dosage Form : Gtt Opht
Dosage Strength :
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Alphagan
Dosage Form : EYE DROPS, SOLUTION
Dosage Strength : 0.2% (2 MG / ML)
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Several substances refer to content
Brand Name : Combigan
Dosage Form : EYE DROPS, SOLUTION
Dosage Strength : 2 MG / ML + 5 MG / ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Alphagan
Dosage Form : Brimonidine 0.2% 5Ml Solution Ophthalmic Use
Dosage Strength : EYE DROPS 5 ml 2 mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Alphagan
Dosage Form : Eye drops, resolution
Dosage Strength : 2 mg/ml
Packaging : Bottle of plastic with dropping tip
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : ALPHAGAN
Dosage Form : SOLUTION
Dosage Strength : 0.2%/W/V
Packaging :
Approval Date :
Application Number : 2236876
Regulatory Info : Prescription
Registration Country : Canada
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : COMBIGAN
Dosage Form : SOLUTION
Dosage Strength : 0.2%/W/V
Packaging :
Approval Date :
Application Number : 2248347
Regulatory Info : Prescription
Registration Country : Canada
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : ALPHAGAN P
Dosage Form : SOLUTION
Dosage Strength : 0.15%/W/V
Packaging :
Approval Date :
Application Number : 2248151
Regulatory Info : Prescription
Registration Country : Canada
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : BRIMONIDINE P
Dosage Form : SOLUTION
Dosage Strength : 0.15%/W/V
Packaging : 5ML/10ML
Approval Date :
Application Number : 2301334
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : APO-BRIMONIDINE-TIMOP
Dosage Form : SOLUTION
Dosage Strength : 0.2%
Packaging : 5ML/10ML
Approval Date :
Application Number : 2375311
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : LUMIFY
Dosage Form : SOLUTION
Dosage Strength : 0.025%/W/V
Packaging :
Approval Date :
Application Number : 2517698
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : MED-BRIMONIDINE
Dosage Form : SOLUTION
Dosage Strength : 0.2%/W/V
Packaging :
Approval Date :
Application Number : 2507811
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : BRIMONIDINE TARTRATE OPHTHALMIC SOLUTION
Dosage Form : SOLUTION
Dosage Strength : 0.2%/W/V
Packaging :
Approval Date :
Application Number : 2515377
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : SIMBRINZA
Dosage Form : SUSPENSION
Dosage Strength : 0.2%/W/V
Packaging : 10ML
Approval Date :
Application Number : 2435411
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic Dossiers Registered in at Least one EU Member State
Registration Country : Canada
Brand Name :
Dosage Form : OPHTHALMIC DROPS
Dosage Strength : 0.2%
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic Dossiers Registered in at Least one EU Member State
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Alphagan P 1.5
Dosage Form : eye drops
Dosage Strength : 0.15%
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Enidin
Dosage Form : eye drops
Dosage Strength : 0.2%
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Alphagan
Dosage Form : eye drops
Dosage Strength : 0.2%
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Alphagan P 1.5
Dosage Form : eye drops
Dosage Strength : 0.15%
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Enidin
Dosage Form : eye drops
Dosage Strength : 0.2%
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Alphagan
Dosage Form : eye drops
Dosage Strength : 0.2%
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Alphagan Purite
Dosage Form : OPD
Dosage Strength : 4mg/ml
Packaging : 5X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Agobrim Eye Drops
Dosage Form : OPD
Dosage Strength : 2mg/ml
Packaging : 5X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : BRIMOCT
Dosage Form : OPD
Dosage Strength : 2mg/ml
Packaging : 5X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : BRIMOCT CO
Dosage Form : OPD
Dosage Strength : 2mg/ ml
Packaging : 5X1mg/ ml
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Simbrinza
Dosage Form : OPD
Dosage Strength : 2mg/ml
Packaging : 5X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
30 May 2022
Reply
18 Feb 2020
Reply
15 Feb 2020
Reply
18 Nov 2019
Reply
19 Jul 2019

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
Patent Expiration Date : 2024-03-02
US Patent Number : 8858961*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 21770
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2024-03-02
Patent Expiration Date : 2030-06-17
BRIMONIDINE TARTRATE; BRINZOLAMIDE
US Patent Number : 9421265
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 204251
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-06-17

Patent Expiration Date : 2030-10-30
BRIMONIDINE TARTRATE; BRINZOLAMIDE
US Patent Number : 9044484
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 204251
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-10-30

Patent Expiration Date : 2030-07-14
US Patent Number : 9259425
Drug Substance Claim :
Drug Product Claim :
Application Number : 218424
Patent Use Code : U-2222
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-07-14

Patent Expiration Date : 2029-07-27
US Patent Number : 11596600
Drug Substance Claim :
Drug Product Claim :
Application Number : 218424
Patent Use Code : U-2222
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2029-07-27

Patent Expiration Date : 2031-03-25
US Patent Number : 9861631
Drug Substance Claim :
Drug Product Claim :
Application Number : 204708
Patent Use Code : U-1428
Delist Requested :
Patent Use Description : TOPICAL TREATMENT OF F...
Patent Expiration Date : 2031-03-25

Patent Expiration Date : 2031-06-13
US Patent Number : 10201517
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 204708
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2031-06-13

Patent Expiration Date : 2025-08-25
US Patent Number : 7439241
Drug Substance Claim :
Drug Product Claim :
Application Number : 204708
Patent Use Code : U-1428
Delist Requested :
Patent Use Description : TOPICAL TREATMENT OF F...
Patent Expiration Date : 2025-08-25

Patent Expiration Date : 2025-05-24
US Patent Number : 8231885
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 204708
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2025-05-24

Patent Expiration Date : 2031-03-25
US Patent Number : 9861632
Drug Substance Claim :
Drug Product Claim :
Application Number : 204708
Patent Use Code : U-1428
Delist Requested :
Patent Use Description : TOPICAL TREATMENT OF F...
Patent Expiration Date : 2031-03-25

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?