
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
FDF
0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
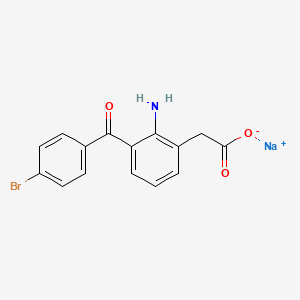

1. Ahr-10282
2. Bromfenac
3. Duract
4. Isv-303
5. Sodium Bromfenac
6. Xibrom
1. 91714-93-1
2. 120638-55-3
3. Bromfenac (sodium)
4. Bronuck
5. Bromfenac Sodium Salt
6. Bromsite
7. Bromfenac Sodium Anhydrous
8. Xibrom
9. Chebi:140536
10. Ahr 10282b
11. Bromfenac Monosodium Salt Sesquihydrate
12. Bromfenac (sodium Hydrate)
13. Benzeneacetic Acid, 2-amino-3-(4-bromobenzoyl)-, Monosodium Salt
14. Sodium;2-[2-amino-3-(4-bromobenzoyl)phenyl]acetate
15. 9x8yf771ou
16. Bromday
17. Prolensa
18. Sodium 2-(2-amino-3-(4-bromobenzoyl)phenyl)acetate
19. Ahr-10282b
20. Bromfenac Sodium Hydrate
21. Sodium 2-[2-amino-3-(4-bromobenzoyl)phenyl]acetate
22. 2-amino-3-(4-bromobenzoyl)benzeneacetic Acid Sodium Salt
23. Bromfenac Sodium Salt Sesquihydrate
24. Unii-9x8yf771ou
25. Sodium [2-amino-3-(4-bromobenzoyl)phenyl]acetate
26. Mfcd03701673
27. Benzeneacetic Acid, 2-amino-3-(4-bromobenzoyl)-, Sodium Salt (1:1)
28. Bromfenac Monosodium Salt
29. Chembl751
30. Schembl56644
31. Hy-b1888a
32. Dtxsid70273981
33. Bromfenac Sodium [who-dd]
34. Ahr 10282r
35. Hms3652d21
36. Hms3885l13
37. Amy22137
38. Bcp05811
39. Bromfenac Sodium, >=98% (hplc)
40. Ac-149
41. Bdbm50225143
42. S4248
43. Akos000280377
44. Bromfenac Monosodium Salt [mi]
45. Ac-9334
46. Ccg-268104
47. Sb17363
48. Bb164264
49. Cs-0014295
50. Ft-0602701
51. Ft-0660252
52. Sw219148-1
53. Sodium 2-amino-3-(4-bromobenzoyl) Phenylacetate
54. J-004372
55. Q-200752
56. Sodium2-(2-amino-3-(4-bromobenzoyl)phenyl)acetate
57. Q27225685
58. Sodium (-amino-3-(p-bromobenzoyl)phenyl)acetate
59. Sodium; [2-amino-3-(4-bromo-benzoyl)-phenyl]-acetate
60. Benzeneacetic Acid,2-amino-3-(4-bromobenzoyl)-,monosodium Salt
61. Ahr 10282b;ahr-10282; Ahr10282; Ahr 10282;sodium Bromfenac
| Molecular Weight | 356.15 g/mol |
|---|---|
| Molecular Formula | C15H11BrNNaO3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 354.98200 g/mol |
| Monoisotopic Mass | 354.98200 g/mol |
| Topological Polar Surface Area | 83.2 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 372 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Bromfenac sodium |
| Drug Label | Bromfenac ophthalmic solution 0.09% is a sterile, topical, nonsteroidal anti-inflammatory drug (NSAID) for ophthalmic use. Each mL of bromfenac ophthalmic solution contains 1.035 mg bromfenac sodium (equivalent to 0.9 mg bromfenac free acid). Bromfen... |
| Active Ingredient | Bromfenac sodium |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | eq 0.09% acid |
| Market Status | Prescription |
| Company | Apotex; Hi-tech Pharmacal; Luitpold; Coastal Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Bromfenac sodium |
| Drug Label | Bromfenac ophthalmic solution 0.09% is a sterile, topical, nonsteroidal anti-inflammatory drug (NSAID) for ophthalmic use. Each mL of bromfenac ophthalmic solution contains 1.035 mg bromfenac sodium (equivalent to 0.9 mg bromfenac free acid). Bromfen... |
| Active Ingredient | Bromfenac sodium |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | eq 0.09% acid |
| Market Status | Prescription |
| Company | Apotex; Hi-tech Pharmacal; Luitpold; Coastal Pharms |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
 Farmak works in the development, production and marketing of APIs, Intermediates & Specialties// FDA inspected.
Farmak works in the development, production and marketing of APIs, Intermediates & Specialties// FDA inspected.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38922
Submission : 2023-10-18
Status : Active
Type : II

Registration Number : 230MF10078
Registrant's Address : Na vlcinci 16/3 Klasterni Hradisko 77900 Olomouc Czech Republic
Initial Date of Registration : 2018-07-03
Latest Date of Registration : --
 ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-10-17
Pay. Date : 2013-07-09
DMF Number : 24521
Submission : 2011-05-20
Status : Active
Type : II
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.

GDUFA
DMF Review : Reviewed
Rev. Date : 2014-01-27
Pay. Date : 2014-01-17
DMF Number : 23653
Submission : 2010-03-15
Status : Active
Type : II

Registration Number : 223MF10031
Registrant's Address : 207, Sujeong-ro, Jangan-myeon, Hwaseong-si, Gyeonggi-do, 18581, Republic of Korea
Initial Date of Registration : 2011-03-03
Latest Date of Registration : --


GDUFA
DMF Review : Reviewed
Rev. Date : 2013-10-09
Pay. Date : 2013-08-28
DMF Number : 25245
Submission : 2011-08-18
Status : Active
Type : II
Date of Issue : 2019-07-15
Valid Till : 2022-07-15
Written Confirmation Number : WC-0152
Address of the Firm :

NDC Package Code : 14445-008
Start Marketing Date : 2013-12-18
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-04-04
Pay. Date : 2013-12-23
DMF Number : 27800
Submission : 2013-12-23
Status : Active
Type : II
Date of Issue : 2019-08-16
Valid Till : 2022-08-15
Written Confirmation Number : WC-0225
Address of the Firm :

NDC Package Code : 42571-281
Start Marketing Date : 2018-10-30
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (10kg/10kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-04-23
Pay. Date : 2013-09-20
DMF Number : 25315
Submission : 2011-11-15
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16414
Submission : 2003-02-07
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 10590
Submission : 1993-11-22
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Farmak works in the development, production and marketing of APIs, Intermediates & Specialties// FDA inspected.
Farmak works in the development, production and marketing of APIs, Intermediates & Specialties// FDA inspected.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38922
Submission : 2023-10-18
Status : Active
Type : II
 ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
GDUFA
DMF Review : Complete
Rev. Date : 2013-10-17
Pay. Date : 2013-07-09
DMF Number : 24521
Submission : 2011-05-20
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22915
Submission : 2009-06-30
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-05-01
Pay. Date : 2013-04-16
DMF Number : 23031
Submission : 2009-07-31
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-10-09
Pay. Date : 2013-08-28
DMF Number : 25245
Submission : 2011-08-18
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 10590
Submission : 1993-11-22
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-04-04
Pay. Date : 2013-12-23
DMF Number : 27800
Submission : 2013-12-23
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16414
Submission : 2003-02-07
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-04-23
Pay. Date : 2013-09-20
DMF Number : 25315
Submission : 2011-11-15
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-01-27
Pay. Date : 2014-01-17
DMF Number : 23653
Submission : 2010-03-15
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
CAS Number : 91714-50-0
End Use API : Bromfenac Sodium
About The Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our cor...
3-chloro-7-(4-bromobenzoyl)-indole
CAS Number : 91714-51-1
End Use API : Bromfenac Sodium
About The Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our cor...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Application : Co-Processed Excipients
Excipient Details : DiCOM-DC S604 is used as a DC co-processed excipient with a combination of various polymers that act as fillers and binders in tablets and capsules.
Pharmacopoeia Ref : In-house
Technical Specs : Polyvinyl Pyrrolidone 97% & 93%
Ingredient(s) : Calcium Carbonate Excipient
Dosage Form : Capsule
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Reply
09 Jul 2024
Reply
17 Sep 2021
Reply
10 Aug 2020
Reply
06 Jun 2019
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?