
Synopsis
Synopsis
0
VMF
0
Canada

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
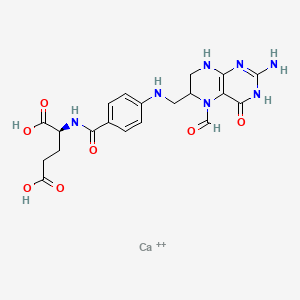

1. 5 Formyltetrahydrofolate
2. 5 Formyltetrahydropteroylglutamate
3. 5-formyltetrahydrofolate
4. 5-formyltetrahydropteroylglutamate
5. Acid, Folinic
6. Calcium Folinate
7. Citrovorum Factor
8. Factor, Citrovorum
9. Folinate, Calcium
10. Folinic Acid
11. Folinic Acid Sf
12. Folinic Acid-sf
13. Leucovorin
14. Leucovorin, (d)-isomer
15. Leucovorin, (dl)-isomer
16. Leucovorin, (r)-isomer
17. Leucovorin, Calcium
18. Leucovorin, Calcium (1:1) Salt
19. Leucovorin, Calcium (1:1) Salt, (dl)-isomer
20. Leucovorin, Calcium (1:1) Salt, Pentahydrate
21. Leucovorin, Monosodium Salt
22. Leukovorin
23. Leukovorum
24. Monosodium Salt Leucovorin
25. N(5)-formyltetrahydrofolate
26. Wellcovorin
1. Calcium Folinate
2. Calcium Citrovorum Factor
3. Folinic Acid Calcium Salt
4. Folinic Acid-sf, Calcium Salt
5. (+)-l-folinic Acid, Calcium Salt
6. 1492-18-8
7. Nsc3590
8. Sr-05000001662
9. Pharmakon1600-01500364
10. Nsc757083
11. Nsc-757083
12. Sr-05000001662-1
13. Sr-05000001662-2
14. Glutamic Acid,6,7,8-tetrahydro-4-hydroxy-6- Pteridinyl)methyl]amino]benzoyl]-, Calcium Salt (1:1), L-
15. L-glutamic Acid,4,5,6,7,8-hexahydro-4-oxo-6- Pteridinyl)methyl]amino]benzoyl]-, Calcium Salt (1:1), (s)-
| Molecular Weight | 513.5 g/mol |
|---|---|
| Molecular Formula | C20H23CaN7O7+2 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 9 |
| Exact Mass | 513.1284869 g/mol |
| Monoisotopic Mass | 513.1284869 g/mol |
| Topological Polar Surface Area | 216 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 2 |
| Complexity | 911 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antidotes
Agents counteracting or neutralizing the action of POISONS. (See all compounds classified as Antidotes.)
Vitamin B Complex
A group of water-soluble vitamins, some of which are COENZYMES. (See all compounds classified as Vitamin B Complex.)
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AF - Detoxifying agents for antineoplastic treatment
V03AF03 - Calcium folinate
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
About the Company : Established in 1994, Rochem is a distributor of pharmaceutical, food, nutritional and animal health ingredients to some of the largest companies in the world. It sources high-quali...
About the Company : Allastir instituted the services in 2010 with a motive to cater the pharmaceutical industry with niche API's. In Allastir we strongly believe in team work and customer satisfaction...

About the Company : Anthem Biosciences is a leading contract research and innovation service provider (CRISP) in Bengaluru, India, which manufactures novel drug actives. Established in 2007, it specia...

About the Company : Cerbios is a privately held company located in Lugano, Switzerland. Cerbios is the ideal CDMO partner from clinical to commercial supply of ADCs, HPAPIs, Proteins and Antibodies. ...

About the Company : In the dinamic pharmaceutical field, DEAFARMA is the reference point for primaries Pharmaceutical Laboratories for over twenty years, even in the national and international territo...

About the Company : As an EU-GMP certified global company and an established hallmark for pharmaceutical standards, Global Calcium has stood the test of time since its inception in 1979 as Calcium Ind...

About the Company : HENGDIAN GROUP was established in 1975, till now it has become a transnational,conglomerate group and been one of the largest private-owned enterprises in China. The Pharmaceutical...

About the Company : Established in the year 2004, Sakar is engaged in manufacturing of Pharmaceutical products providing Liquid Orals, Cephalosporin Tablet, Capsule, Dry Powder Syrup, Dry Powder Injec...

About the Company : Shouyuan chemical (one of the leading chemicals supplier in China) specializes in manufacturing, supplying, and custom synsthesis latest chemicals. Our products cover all kinds of ...

About the Company : Huiyu Pharma, established in Oct. 2010, is a specialized pharmaceutical company focusing on developing and manufacturing high-quality oncology products. At present, products in our...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
9,10-Dehydro Folitixorin Chloride
CAS Number : 804563-04-0
End Use API : Calcium Folinate
About The Company : Fermion is fully owned subsidiary of Orion Corporation & headquartered in Espoo, Finland. Together with Orion we are a fully integrated CDMO & offer services co...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 5MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Regulatory Info :
Registration Country : France
Brand Name :
Dosage Form : Tablet
Dosage Strength : 5MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : France
 Ethypharm is an international Pharma company with European roots manufacturing and commercializing essential drugs all over the world.
Ethypharm is an international Pharma company with European roots manufacturing and commercializing essential drugs all over the world.
Regulatory Info :
Registration Country : France
Brand Name :
Dosage Form : Vial
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : France
 Ethypharm is an international Pharma company with European roots manufacturing and commercializing essential drugs all over the world.
Ethypharm is an international Pharma company with European roots manufacturing and commercializing essential drugs all over the world.
Regulatory Info :
Registration Country : France
Brand Name :
Dosage Form : Vial
Dosage Strength : 350MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : France
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 100MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 200MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 350MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 100MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 200MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : LEUCOVORIN CALCIUM PRESERVATIVE FREE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 500MG BASE/VIAL
Approval Date : 1999-02-26
Application Number : 40286
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : LEUCOVORIN CALCIUM
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 5MG BASE
Approval Date : 1993-02-22
Application Number : 72733
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : LEUCOVORIN CALCIUM
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 10MG BASE
Approval Date : 1993-02-22
Application Number : 72734
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : LEUCOVORIN CALCIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 350MG BASE/VIAL **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 1989-04-05
Application Number : 8107
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : LEUCOVORIN CALCIUM
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 15MG BASE
Approval Date : 2020-10-22
Application Number : 213929
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : LEUCOVORIN CALCIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 50MG BASE/VIAL
Approval Date : 1997-04-17
Application Number : 89628
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : LEUCOVORIN CALCIUM
Dosage Form : Injectable; Injection
Dosage Strength : EQ 5MG BASE/ML
Approval Date :
Application Number : 89504
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : LEUCOVORIN CALCIUM PRESERVATIVE FREE
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS
Dosage Strength : EQ 500MG BASE/50ML (EQ 10MG BASE/ML)
Approval Date : 1999-06-28
Application Number : 40332
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : LEUCOVORIN CALCIUM PRESERVATIVE FREE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 100MG BASE/VIAL
Approval Date : 2017-05-19
Application Number : 203800
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : LEUCOVORIN CALCIUM PRESERVATIVE FREE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 200MG BASE/VIAL
Approval Date : 2017-05-19
Application Number : 203800
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?