
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
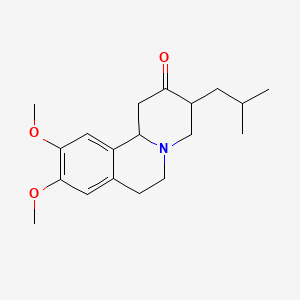

1. Nitoman
2. Xenazine
1. 58-46-8
2. Nitoman
3. 718635-93-9
4. Rubigen
5. Tetrabenzaine
6. Tetrabenzine
7. Tetra Benazin
8. Xenazine
9. Tetrabenazine (racemate)
10. 3-isobutyl-9,10-dimethoxy-3,4,6,7-tetrahydro-1h-pyrido[2,1-a]isoquinolin-2(11bh)-one
11. Tetrabenazine Racemate
12. Ro 1-9569 Racemate
13. Tetrabenazinum
14. Ro 1-9569
15. Tetrabenazinum [inn-latin]
16. Tetrabenazina [inn-spanish]
17. 9,10-dimethoxy-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydrobenzo[a]quinolizin-2-one
18. 9,10-dimethoxy-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-one
19. Deutetrabenazine
20. 9,10-dimethoxy-3-(2-methylpropyl)-1h,2h,3h,4h,6h,7h,11bh-pyrido[2,1-a]isoquinolin-2-one
21. Mls003106727
22. Ro 1-9569/12
23. Chembl117785
24. Chebi:64028
25. (-)-tetrabenazine
26. Ro-19569
27. 2-oxo-3-isobutyl-9,10-dimethoxy-1,2,3,4,6,7-hexahydro-11bh-benzo[a]quinolizine
28. 9,10-dimethoxy-3-isobutyl-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-one
29. Nsc-169886
30. Ncgc00160421-01
31. Nsc 169886
32. 1,2,4,6,7,11b-hexahydro-3-isobutyl-9,10-dimethoxy-2h-benzo[a]quinolizin-2-one
33. 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-one
34. Dsstox_cid_22614
35. Dsstox_rid_80059
36. Dsstox_gsid_42614
37. 2-oxo-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-benzoquinolizine
38. Cas-58-46-8
39. Xenazine (tn)
40. Smr000718664
41. Nitoman (tn)
42. Wln: T B666 Dv Gntt&j E1y1&1 Lo1 Mo1
43. Tetrabenazin
44. Revocon
45. 2-oxo-3-isobutyl-9,2,3,4,6,7-hexahydro-11bh-benzo[a]quinolizine
46. 2-oxo-3-isobutyl-9,3,4,6,7,11.beta.-hexahydro-2h-benzoquinolizine
47. 1,4,6,7,11b-hexahydro-3-isobutyl-9,10-dimethoxy-2h-benzo[a]quinolizin-2-one
48. 2h-benzo[a]quinolizin-2-one,3,4,6,7,11b-hexahydro-3-isobutyl-9,10-dimethoxy-
49. 2h-benzo[a]quinolizin-2-one,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-
50. 2h-benzo(a)quinolizin-2-one, 1,3,4,6,7,11b-hexahydro-3-isobutyl-9,10-dimethoxy-
51. 2h-benzo[a]quinolizin-2-one, 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-
52. Ro-1-9569
53. Tetrabenazine- Bio-x
54. Nitoman;ro 1-9569
55. Tetrabenazine (jan/inn)
56. Timtec1_002217
57. 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2h-benzo[a]quinolizin-2-one
58. Oprea1_264344
59. Schembl62026
60. Mls001249426
61. Mls001249497
62. Gtpl4834
63. Hy-b0590a
64. Tetrabenazine Related Impurity 2
65. Dtxsid501127442
66. Hms1540e17
67. Hms2966e06
68. Hms3263i14
69. Hms3430f03
70. Hms3884e10
71. Tetrabenazine (nitoman, Xenazine)
72. Act06890
73. Bcp15831
74. Bcp24156
75. Ex-a2361
76. Tox21_111803
77. Tox21_501086
78. Bdbm50017701
79. Mfcd00042740
80. Mfcd11519951
81. Nsc169886
82. Nsc172187
83. S1789
84. Stk678061
85. Akos001681311
86. Akos016347602
87. Tox21_111803_1
88. Ccg-118217
89. Cs-5700
90. Db04844
91. Lp01086
92. Nsc-172187
93. Pb21652
94. Pb25061
95. Pb38735
96. Sb49797
97. Sdccgsbi-0633786.p001
98. Ncgc00160421-02
99. Ncgc00160421-03
100. Ncgc00160421-06
101. Ncgc00261771-01
102. As-35785
103. Bt164463
104. Sy055407
105. Ft-0674921
106. Ft-0700948
107. Ft-0771873
108. Ft-0772314
109. D08575
110. A856325
111. A917912
112. Q413050
113. Sr-01000833864
114. Sr-01000833864-4
115. Brd-a47564106-001-01-8
116. Z1563146114
117. 2-oxo-3-isobutyl-9,10-dimethoxy-1,2,3,4,6,7,11b-hexahydro-2h-benzo(a)quinolizine
118. 2h-benzo[a]quinolizin-2-one, 1,3,4,6,7, 11b-hexahydro-3-isobutyl-9,10-dimethoxy-,
119. 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-one #
120. 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-pyrido[2,1-a]isoquinolin-2-one
121. 3-isobutyl-9-,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-benzo[a]quinolizin-2-one
122. Tetrabenazine (3r,11br)-rel-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2h-benzo[a]quinolizin-2-one
| Molecular Weight | 317.4 g/mol |
|---|---|
| Molecular Formula | C19H27NO3 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 317.19909372 g/mol |
| Monoisotopic Mass | 317.19909372 g/mol |
| Topological Polar Surface Area | 38.8 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 425 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Xenazine |
| PubMed Health | Tetrabenazine (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Drug Label | XENAZINE (tetrabenazine) is a monoamine depletor for oral administration. The molecular weight of tetrabenazine is 317.43; the pKa is 6.51. Tetrabenazine is a hexahydro-dimethoxy-benzoquinolizine derivative and has the following chemical name: cis ra... |
| Active Ingredient | Tetrabenazine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 12.5mg |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 2 of 2 | |
|---|---|
| Drug Name | Xenazine |
| PubMed Health | Tetrabenazine (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Drug Label | XENAZINE (tetrabenazine) is a monoamine depletor for oral administration. The molecular weight of tetrabenazine is 317.43; the pKa is 6.51. Tetrabenazine is a hexahydro-dimethoxy-benzoquinolizine derivative and has the following chemical name: cis ra... |
| Active Ingredient | Tetrabenazine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 12.5mg |
| Market Status | Prescription |
| Company | Valeant Bermuda |
Adrenergic Uptake Inhibitors
National Library of Medicine's Medical Subject Headings. Tetrabenazine. Online file (MeSH, 2018). Available from, as of March 7, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Tetrabenazine is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 7, 2018: https://clinicaltrials.gov/
Xenazine is indicated for the treatment of chorea associated with Huntington's disease. /Included in US product label/
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
/EXPL THER/ Since levodopa-induced peak dyskinesias (LIDs) may reflect, in part, a disproportionate phasic release of dopamine from synaptic vesicles, we examined the ability of the vesicular depletor tetrabenazine (TBZ) to reduce LIDs in 10 dyskinetic advanced Parkinson's disease (PD) patients. After basal evaluation, the patients received, through a slow titration, oral TBZ twice a day for six weeks (up to 50 mg daily) before being re-assessed after a challenge with levodopa. The primary outcome measure was the change in the Unified Parkinson's Disease Rating Scale (UPDRS) dyskinesia score (items 32 to 34). TBZ was well tolerated. A clear treatment effect on LIDs emerged (up to 45%, p<0.05). In two patients a little worsening of motor performance necessitated an increase of the antiparkinsonian therapy, which did not worsen peak-dose LIDs. The patients experienced a clear benefit in terms of their quality of life. In this open-label pilot study, orally administered TBZ resulted in objective and subjective improvements in LIDs. Larger pharmacological studies are in progress.
PMID:24125559 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3812731 Brusa L et al; Funct Neurol. 2013 Apr-May;28(2):101-5 (2013)
For more Therapeutic Uses (Complete) data for Tetrabenazine (8 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: DEPRESSION AND SUICIDALITY. Xenazine can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington's disease. Anyone considering the use of Xenazine must balance the risks of depression and suicidality with the clinical need for control of chorea. Close observation of patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior should accompany therapy. Patients, their caregivers, and families should be informed of the risk of depression and suicidality and should be instructed to report behaviors of concern promptly to the treating physician. Particular caution should be exercised in treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in Huntington's disease. Xenazine is contraindicated in patients who are actively suicidal, and in patients with untreated or inadequately treated depression.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
Caution should be exercised in treating patients with tetrabenazine who have a history of depression or prior suicide attempts or ideation since these patients may be at an increased risk for suicidal behavior. Patients who are actively suicidal or those with untreated or inadequately treated depression should not be treated with the drug.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
Huntington's disease is a progressive disorder characterized by changes in mood, cognition, chorea, rigidity, and functional capacity over time. In a 12-week controlled trial, Xenazine was also shown to cause slight worsening in mood, cognition, rigidity, and functional capacity. Whether these effects persist, resolve, or worsen with continued treatment is unknown.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
Prior to administering tetrabenazine dosages exceeding 50 mg daily, the manufacturer recommends patients be tested to determine their cytochrome P-450 (CYP) isoenzyme 2D6 status (i.e., poor metabolizers, extensive metabolizers, intermediate metabolizers).1 Drug exposure will be substantially higher (about threefold for alpha-dihydrotetrabenazine (a-HTBZ) and ninefold for beta-dihydrotetrabenazine (beta-HTBZ), both active metabolites) when a dose is given to a poor metabolizer than when given to an extensive metabolizer.1 The manufacturer recommends limiting the dosage of tetrabenazine to 50 mg daily and single doses of the drug to 25 mg in patients who are poor CYP2D6 metabolizers.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
For more Drug Warnings (Complete) data for Tetrabenazine (22 total), please visit the HSDB record page.
Treatment of hyperkinetic movement disorders like chorea in Huntington's disease, hemiballismus, senile chorea, Tourette syndrome and other tic disorders, and tardive dyskinesia
FDA Label
Prolongation of the QTc interval has been observed at doses of 50 mg. In rats, it has been observed that tetrabenazine or its metabolites bind to melanin-containing tissues such as the eyes and skin. After a single oral dose of radiolabeled tetrabenazine, radioactivity was still detected in eye and fur at 21 days post dosing.
Adrenergic Uptake Inhibitors
Drugs that block the transport of adrenergic transmitters into axon terminals or into storage vesicles within terminals. The tricyclic antidepressants (ANTIDEPRESSIVE AGENTS, TRICYCLIC) and amphetamines are among the therapeutically important drugs that may act via inhibition of adrenergic transport. Many of these drugs also block transport of serotonin. (See all compounds classified as Adrenergic Uptake Inhibitors.)
N - Nervous system
N07 - Other nervous system drugs
N07X - Other nervous system drugs
N07XX - Other nervous system drugs
N07XX06 - Tetrabenazine
N - Nervous system
N07 - Other nervous system drugs
N07X - Other nervous system drugs
N07XX - Other nervous system drugs
N07XX16 - Deutetrabenazine
Absorption
Following oral administration of tetrabenazine, the extent of absorption is at least 75%. After single oral doses ranging from 12.5 to 50 mg, plasma concentrations of tetrabenazine are generally below the limit of detection because of the rapid and extensive hepatic metabolism of tetrabenazine. Food does not affect the absorption of tetrabenazine. Cmax, oral = 4.8 ng/mL in HD or tardive dyskinesia patients; Tmax, oral = 69 min in HD or tardive dyskinesia patients
Route of Elimination
After oral administration, tetrabenazine is extensively hepatically metabolized, and the metabolites are primarily renally eliminated (75%). Tetrabenazine is also cleared fecally (7% to 16%). Unchanged tetrabenazine has not been found in human urine. Urinary excretion of -HTBZ or -HTBZ (the major metabolites) accounted for less than 10% of the administered dose.
Volume of Distribution
Steady State, IV, in HD or tardive dyskinesia patients: 385L. Tetrabenazine is rapidly distributed to the brain following IV injection. The site with the highest binding is the striatum, while the lowest binding was observed in the cortex.
Clearance
IV, 1.67 L/min in HD or tardive dyskinesia patients
The in vitro protein binding of tetrabenazine, alpha-dihydrotetrabenazine (a-HTBZ), and beta-dihydrotetrabenazine (b-HTBZ) was examined in human plasma for concentrations ranging from 50 to 200 ng/mL. Tetrabenazine binding ranged from 82% to 85%, a-HTBZ binding ranged from 60% to 68%, and b-HTBZ binding ranged from 59% to 63%.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
Results of PET-scan studies in humans show that radioactivity is rapidly distributed to the brain following intravenous injection of (11)C-labeled tetrabenazine or alpha-dihydrotetrabenazine (a-HTBZ), with the highest binding in the striatum and lowest binding in the cortex.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
Tetrabenazine or its metabolites bind to melanin-containing tissues (i.e., eye, skin, fur) in pigmented rats. After a single oral dose of radiolabeled tetrabenazine, radioactivity was still detected in eye and fur at 21 days post dosing.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
In a mass balance study in 6 healthy volunteers, approximately 75% of the dose was excreted in the urine, and fecal recovery accounted for approximately 7 to 16% of the dose. Unchanged tetrabenazine has not been found in human urine.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
Following oral administration of tetrabenazine, the extent of absorption is at least 75%. After single oral doses ranging from 12.5 to 50 mg, plasma concentrations of tetrabenazine are generally below the limit of detection because of the rapid and extensive hepatic metabolism of tetrabenazine by carbonyl reductase to the active metabolites alpha-dihydrotetrabenazine (a-HTBZ) and beta-dihydrotetrabenazine (b-HTBZ). a-HTBZ and b-HTBZ are metabolized principally by CYP2D6. Peak plasma concentrations (Cmax) of a-HTBZ and b-HTBZ are reached within 1 to 1 1/2 hours post-dosing. a-HTBZ is subsequently metabolized to a minor metabolite, 9-desmethyl-a-DHTBZ. b-HTBZ is subsequently metabolized to another major circulating metabolite, 9-desmethyl-b-DHTBZ, for which Cmax is reached approximately 2 hours post-dosing.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
Tetrabenazine is hepatically metabolized. Carbonyl reductase in the liver is responsible for the formation of two major active metabolites: -dihydrotetrabenazine (-HTBZ) and -dihydrotetrabenazine (-HTBZ). -HTBZ is further metabolized into 9-desmethyl--DHTBZ, a minor metabolite by CYP2D6 and with some contribution of CYP1A2. -HTBZ is metabolized to another major circulating metabolite, 9-desmethyl--DHTBZ, by CYP2D6. The Tmax of this metabolite is 2 hours post-administration of tetrabenazine.
In a mass balance study in 6 healthy volunteers, approximately 75% of the dose was excreted in the urine, and fecal recovery accounted for approximately 7 to 16% of the dose. Unchanged tetrabenazine has not been found in human urine. Urinary excretion of alpha-dihydrotetrabenazine (a-HTBZ) or beta-dihydrotetrabenazine (b-HTBZ) accounted for less than 10% of the administered dose. Circulating metabolites, including sulfate and glucuronide conjugates of HTBZ metabolites as well as products of oxidative metabolism, account for the majority of metabolites in the urine.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
After oral administration, tetrabenazine is extensively hepatically metabolized, and the metabolites are primarily renally eliminated.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
The results of in vitro studies do not suggest that tetrabenazine, alpha-dihydrotetrabenazine (a-HTBZ), beta-dihydrotetrabenazine (b-HTBZ) or 9-desmethyl-beta-dihydrotetrabenazine are likely to result in clinically significant inhibition of CYP2D6, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, or CYP3A. In vitro studies suggest that neither tetrabenazine nor its a- or b-HTBZ or 9-desmethyl-beta-dihydrotetrabenazine metabolites are likely to result in clinically significant induction of CYP1A2, CYP3A4, CYP2B6, CYP2C8, CYP2C9, or CYP2C19.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
After oral administration in humans, at least 19 metabolites of tetrabenazine have been identified. alpha-Dihydrotetrabenazine (a-HTBZ), beta-dihydrotetrabenazine (b-HTBZ) and 9-desmethyl-beta-dihydrotetrabenazine are the major circulating metabolites and are subsequently metabolized to sulfate or glucuronide conjugates. a-HTBZ and b-HTBZ are formed by carbonyl reductase that occurs mainly in the liver. a-HTBZ is O-dealkylated by CYP450 enzymes, principally CYP2D6, with some contribution of CYP1A2 to form 9-desmethyl-alpha-dihydrotetrabenazine, a minor metabolite. b-HTBZ is O-dealkylated principally by CYP2D6 to form 9-desmethyl-beta-dihydrotetrabenazine.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
Following oral administration of tetrabenazine, the extent of absorption is at least 75%. After single oral doses ranging from 12.5 to 50 mg, plasma concentrations of tetrabenazine are generally below the limit of detection because of the rapid and extensive hepatic metabolism of tetrabenazine by carbonyl reductase to the active metabolites alpha-dihydrotetrabenazine (a-HTBZ) and beta-dihydrotetrabenazine (b-HTBZ). a-HTBZ and b-HTBZ are metabolized principally by CYP2D6. Peak plasma concentrations (Cmax) of a-HTBZ and b-HTBZ are reached within 1 to 1 1/2 hours post-dosing. a-HTBZ is subsequently metabolized to a minor metabolite, 9-desmethyl-a-DHTBZ. b-HTBZ is subsequently metabolized to another major circulating metabolite, 9-desmethyl-b-DHTBZ, for which Cmax is reached approximately 2 hours post-dosing.
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
-HTBZ = 7 hours; -HTBZ = 5 hours; 9-desmethyl--DHTBZ = 12 hours.
alpha-Dihydrotetrabenazine (a-HTBZ), beta-dihydrotetrabenazine (b-HTBZ) and 9-desmethyl-beta-dihydrotetrabenazine have half-lives of 7 hours, 5 hours and 12 hours respectively. /Tetrabenazine metabolites/
NIH; DailyMed. Current Medication Information for Xenazine (Tetrabenazine) Tablet (Updated: September 1, 2017). Available from, as of March 19, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac768bab-8afa-4446-bc7f-caeeffec0cda
Tetrabenazine is a reversible human vesicular monoamine transporter type 2 inhibitor (Ki = 100 nM). It acts within the basal ganglia and promotes depletion of monoamine neurotransmitters serotonin, norepinephrine, and dopamine from stores. It also decreases uptake into synaptic vesicles. Dopamine is required for fine motor movement, so the inhibition of its transmission is efficacious for hyperkinetic movement. Tetrabenazine exhibits weak in vitro binding affinity at the dopamine D2 receptor (Ki = 2100 nM).
... Pharmacology studies demonstrate that betrabenzaine reversibly inhibits the activity of vesicular monoamine transporter 2, resulting in depletion of central dopamine. ...
PMID:22749259 Chen JJ et al; Clin Ther 34 (7): 1487-504 (2012)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
3,4-dihydro-6,7-dimethoxyisoquinolinehydrochloride
CAS Number : 20232-39-7
End Use API : Tetrabenazine
About The Company : Malladi Drugs & Pharmaceuticals Ltd was founded in 1980 by microbiologist Mr. M L N Sastry. Within a few years, Malladi established itself as a leader in manufa...
2-acetyl-N,N,N,4-tetramethylpentan- 1-aminium iodi...
CAS Number : 1069-62-1
End Use API : Tetrabenazine
About The Company : Alkaloids is a family company established in 1967. Starting with production of natural Caffeine, quite a number of speciality products have been added to our p...

6,7-dimethoxy-3,4- dihydroisoquinoline Hydrochlori...
CAS Number : 20232-39-7
End Use API : Tetrabenazine
About The Company : Alkaloids is a family company established in 1967. Starting with production of natural Caffeine, quite a number of speciality products have been added to our p...

3,4-Dimethoxyphenyl acetonitrile (Homoveratryl nit...
CAS Number : 93-17-4
End Use API : Tetrabenazine
About The Company : Established in 2003 with small pilot plant and came in to commercial production in 2013 in the name of Allchem Laboratories, it is an independent privately owne...

3,4-Dimethoxyphenethylamine (Homoveratryl Amine )
CAS Number : 120-20-7
End Use API : Tetrabenazine
About The Company : Established in 2003 with small pilot plant and came in to commercial production in 2013 in the name of Allchem Laboratories, it is an independent privately owne...

6,7-Dimethoxy-3,4-dihydroisoquinoline HCl
CAS Number : 20232-39-7
End Use API : Tetrabenazine
About The Company : Aventus Labs is a forward-thinking pharmaceutical company committed to developing and delivering high-quality, effective healthcare solutions. With a focus on r...

6,7-Dihydroxy-3,4-dihydroisoquinoline
CAS Number : 4602-83-9
End Use API : Tetrabenazine
About The Company : Aventus Labs is a forward-thinking pharmaceutical company committed to developing and delivering high-quality, effective healthcare solutions. With a focus on r...

6,7 Dimethoxy 3,4 Dihydro Isoquinaline HCL
CAS Number : 20232-39-7
End Use API : Tetrabenazine
About The Company : Chemeca Drugs Private Limited was erstwhile M/s. Vegesna Laboratories Pvt Limited. M/s. Vegesna Laboratories Pvt Limited is a Private limited company having its...

3(N,N-Dimethylamino methyl)-5-methyl-2-hexanone
CAS Number : 91342-74-4
End Use API : Tetrabenazine
About The Company : Equilife Laboratories Pvt. Ltd. is an India-based pharmaceutical company, specializing in innovative process development for pharmaceutical products and service...

CAS Number : 93-17-4
End Use API : Tetrabenazine
About The Company : Recordati, established in 1926, is an international pharmaceutical group, listed on the Italian Stock Exchange (Reuters RECI.MI, Bloomberg REC IM, ISIN IT 00038...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Main Therapeutic Indication : CNS & Anesthesia
Currency : USD
2020 Revenue in Millions : 29
2019 Revenue in Millions : 38
Growth (%) : -24

Main Therapeutic Indication : Neurology
Currency : USD
2021 Revenue in Millions : 22
2020 Revenue in Millions : 29
Growth (%) : -24

Main Therapeutic Indication : CNS & Anesthesia
Currency : USD
2019 Revenue in Millions : 38
2018 Revenue in Millions : 52
Growth (%) : -27

Main Therapeutic Indication : Rare Disease
Currency : USD
2015 Revenue in Millions : 254
2014 Revenue in Millions : 330
Growth (%) : 30%

Main Therapeutic Indication : Rare Disease
Currency : USD
2014 Revenue in Millions : 19%
2013 Revenue in Millions :
Growth (%) :

Main Therapeutic Indication : Neurological/Mental Disorders
Currency : USD
2017 Revenue in Millions : 178
2016 Revenue in Millions : 267
Growth (%) : -33

Main Therapeutic Indication : Neurological/Mental Disorders
Currency : USD
2018 Revenue in Millions : 66
2017 Revenue in Millions : 157
Growth (%) : -58%

Main Therapeutic Indication : Rare Diseases
Currency : USD
2016 Revenue in Millions : 157
2015 Revenue in Millions : 223
Growth (%) : -30

Main Therapeutic Indication : Rare Diseases
Currency : USD
2017 Revenue in Millions : 113
2016 Revenue in Millions : 157
Growth (%) : -28

Main Therapeutic Indication : Rare Diseases
Currency : USD
2018 Revenue in Millions : 52
2017 Revenue in Millions : 122
Growth (%) : -57%

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
27 Jun 2022
Reply
17 Apr 2020
Reply
22 Mar 2019
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?