
Synopsis
Synopsis
0
VMF
0
Australia

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
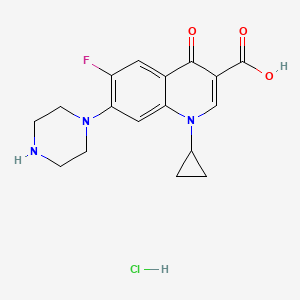

1. Anhydrous, Ciprofloxacin Hydrochloride
2. Bay 09867
3. Bay-09867
4. Bay09867
5. Ciprinol
6. Cipro
7. Ciprofloxacin
8. Ciprofloxacin Hydrochloride Anhydrous
9. Ciprofloxacin Monohydrochloride Monohydrate
10. Hydrochloride Anhydrous, Ciprofloxacin
11. Hydrochloride, Ciprofloxacin
12. Monohydrate, Ciprofloxacin Monohydrochloride
13. Monohydrochloride Monohydrate, Ciprofloxacin
1. Ciprofloxacin Hcl
2. 93107-08-5
3. 86483-48-9
4. 1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic Acid Hydrochloride
5. Cipro
6. Chebi:310388
7. Ciprofloxacin Hydrochloride Anhydrous
8. 0mp32mfp6c
9. 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic Acid Hydrochloride
10. Ciprofloxacin (monohydrochloride)
11. Bay-o-9867
12. Ciprofloxacin Hydrochloride (anh.)
13. Bay O 9867
14. Cetraxal
15. Ciprobay
16. Ciproxan
17. Ciproxin
18. Flociprin
19. Ciflox
20. Mfcd00079044
21. 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic Acid Hcl
22. 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic Acid;hydrochloride
23. 1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic Acid Xhydrochloride
24. Bay-o 9867
25. 93107-08-5 (hcl)
26. 1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydro-3-quinolinecarboxylic Acid Hydrochloride
27. Dsstox_cid_27768
28. Dsstox_rid_82545
29. Dsstox_gsid_47788
30. Ciprofloxacin (as Hydrochloride)
31. Prestwick_67
32. 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-quinoline-3-carboxylic Acid; Hydrochloride
33. 4-(3-carboxy-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-quinolin-7-yl)-piperazin-1-ium; Chloride
34. Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic Acid, Monohydrochloride
35. Nsc620634
36. Ciprofloxacin Hydrochloride (anhydrous)
37. Ciprofloxacin 100 Microg/ml In Methanol
38. Ncgc00016959-01
39. Cas-93107-08-5
40. Unii-0mp32mfp6c
41. 3-quinolinecarboxylic Acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-, Hydrochloride
42. 3-quiolinecarboxylic Acid
43. Ciprofloxacine Hydrochloride
44. Epitope Id:174846
45. Cambridge Id 5807784
46. Ciprofloxacini Hydrochloridum
47. Chembl1202
48. Schembl42310
49. 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic Acid Monohydrochloride
50. 3-quinolinecarboxylic Acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-, Monohydrochloride
51. Ciprofloxacin Hcl/lactate
52. Dtxsid1047788
53. Hy-b0356a
54. Ciprofloxacin Hydrochloride (1:x)
55. Hms1568g08
56. Bcp13634
57. Bcp14336
58. Tox21_110712
59. S5008
60. Akos005111008
61. Tox21_110712_1
62. Cs-8134
63. Ks-5012
64. Nsc-620634
65. Sb73037
66. Ncgc00016959-06
67. 3-quinolinecarboxylic Acid, 1,4-dihydro-1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-, Hydrochloride
68. Ac-23972
69. Bim-0048462.p001
70. Ciprofloxacin 1000 Microg/ml In Methanol
71. Ciprofloxacin Hydrochloride [who-dd]
72. Ft-0623850
73. Ft-0623851
74. A16969
75. H10663
76. A859872
77. Q-200860
78. Q27105154
79. F0001-2378
80. Cpx; Cetraxal; Ciloxan; Cipro; Bay-09867 Hydrochloride
81. 1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic Acid Hcl
82. 1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylicacidxhydrochloride
83. 3-quinolinecarboxylic Acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-, Hydrochloride (1:1)
| Molecular Weight | 367.8 g/mol |
|---|---|
| Molecular Formula | C17H19ClFN3O3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 367.1098973 g/mol |
| Monoisotopic Mass | 367.1098973 g/mol |
| Topological Polar Surface Area | 72.9 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 571 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Ciprofloxacin hydrochloride |
| PubMed Health | Ciprofloxacin Betaine/Ciprofloxacin Hydrochloride (By mouth) |
| Drug Classes | Antibacterial |
| Drug Label | Ciprofloxacin Tablets, USPis a synthetic broad spectrum antimicrobial agent for oral administration. Ciprofloxacin hydrochloride, USP, a fluoroquinolone, is the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-pip... |
| Active Ingredient | Ciprofloxacin hydrochloride |
| Dosage Form | Solution/drops; Tablet |
| Route | Ophthalmic; Oral |
| Strength | eq 100mg base; eq 750mg base; eq 500mg base; eq 0.3% base; eq 250mg base |
| Market Status | Prescription |
| Company | Watson Labs; Nexus Pharms; Ranbaxy; Ivax Sub Teva Pharms; Apotex; Bausch And Lomb; Aurobindo Pharma; Taro; Dr Reddys Labs; Unique Pharm Labs; Carlsbad; Pharmaforce; Fdc; Mylan; Hikma; Akorn |
| 2 of 2 | |
|---|---|
| Drug Name | Ciprofloxacin hydrochloride |
| PubMed Health | Ciprofloxacin Betaine/Ciprofloxacin Hydrochloride (By mouth) |
| Drug Classes | Antibacterial |
| Drug Label | Ciprofloxacin Tablets, USPis a synthetic broad spectrum antimicrobial agent for oral administration. Ciprofloxacin hydrochloride, USP, a fluoroquinolone, is the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-pip... |
| Active Ingredient | Ciprofloxacin hydrochloride |
| Dosage Form | Solution/drops; Tablet |
| Route | Ophthalmic; Oral |
| Strength | eq 100mg base; eq 750mg base; eq 500mg base; eq 0.3% base; eq 250mg base |
| Market Status | Prescription |
| Company | Watson Labs; Nexus Pharms; Ranbaxy; Ivax Sub Teva Pharms; Apotex; Bausch And Lomb; Aurobindo Pharma; Taro; Dr Reddys Labs; Unique Pharm Labs; Carlsbad; Pharmaforce; Fdc; Mylan; Hikma; Akorn |
Treatment of chronic pulmonary infections caused by Pseudomonas aeruginosa
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Cytochrome P-450 CYP1A2 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP1A2. (See all compounds classified as Cytochrome P-450 CYP1A2 Inhibitors.)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?