
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
FDF
0
FDA Orange Book

0
Europe

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
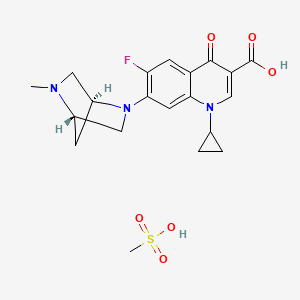

1. Advocid
2. Advocin
3. Danofloxacin
1. 119478-55-6
2. Danofloxacin Mesilate
3. Danofloxacin Methanesulfonate
4. Advocid
5. Danofloxacin (mesylate)
6. Danofloxacin Mesylate [usan]
7. Advocin
8. Cp-76136-27
9. 94f3sx3lem
10. 1-cyclopropyl-6-fluoro-1,4-dihydro-7-((1s,4s)-5-methyl-2,5-diazabicyclo(2.2.1)hept-2-yl)-4-oxo-3-quinolinecarboxylic Acid, Monomethanesulfonate
11. Cp-76,136-27
12. 119478-55-6 (mesylate)
13. Danofloxacin Mesylate (usan)
14. Danofloxacin Mesylate 100 Microg/ml In Acetonitrile
15. 1-cyclopropyl-6-fluoro-7-[(1s,4s)-5-methyl-2,5-diazabicyclo[2.2.1]heptan-2-yl]-4-oxoquinoline-3-carboxylic Acid;methanesulfonic Acid
16. 3-quinolinecarboxylic Acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-7-(5-methyl-2,5-diazabicyclo(2.2.1)hept-2-yl)-4-oxo-, (1s)-, Monomethanesulfonate
17. Danofloxacin Mesilate Hydrate [jan]
18. Unii-94f3sx3lem
19. Advocid (tn)
20. Cp 76,136-27
21. Danofloxacin Mesilate Hydrate
22. Schembl418388
23. Danofloxacin Monomethanesulfonate
24. Chembl2106153
25. Dtxsid30922988
26. Chebi:181474
27. 1-cyclopropyl-6-fluoro-7-((1s,4s)-5-methyl-2,5-diazabicyclo[2.2.1]heptan-2-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Compound With Methanesulfonic Acid (1:1)
28. Hy-b0501
29. Mfcd00673687
30. S3058
31. Danofloxacin Mesilate [mart.]
32. Akos015895387
33. 2,4-dimethylpyrrole-3-carboxylicacid?
34. Ccg-269267
35. 1-cyclopropyl-6-fluoro-1,4-dihydro-7-[(1s,4s)-5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl]-4-oxo-3-quinolinecarboxylic Acid Mesylate
36. As-75249
37. Danofloxacin Methanesulfonate [mi]
38. Danofloxacin Mesylate 100 Microg/ml In Water
39. C71643
40. D-0245
41. D03646
42. Danofloxacin Mesylate 100 Microg/ml In Methanol
43. 478d556
44. Q27271680
45. Danofloxacin Mesylate, Antibiotic For Culture Media Use Only
46. 1-cyclopropyl-6-fluoro-7-((1s,4s)-5-methyl-2,5-diazabicyclo[2.2.1]heptan-2-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Methanesulfonic Acid Salt
47. 1-cyclopropyl-6-fluoro-7-(5-methyl-2,5-diazabicyclo[2.2.1]heptan-2-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid--methanesulfonic Acid (1/1)
48. 1-cyclopropyl-6-fluoro-7-[(1s,4s)-5-methyl-2,5-diazabicyclo[2.2.1]heptan-2-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid; Methanesulfonic Acid
49. 1-cyclopropyl-6-luoro-7-[(1s,4s)-5-methyl-2,5-diazabicyclo[2.2.1]heptan-2-yl]-4-oxoquinoline-3-carboxylic Acid;methanesulonic Acid
50. 3-quinolinecarboxylic Acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-7-[(1s,4s)-5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl]-4-oxo-, Methanesulfonate (1:1)
| Molecular Weight | 453.5 g/mol |
|---|---|
| Molecular Formula | C20H24FN3O6S |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 3 |
| Exact Mass | 453.13698483 g/mol |
| Monoisotopic Mass | 453.13698483 g/mol |
| Topological Polar Surface Area | 127 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 772 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Capsule
Grade : Oral (Pharma Grade)
Application : Fillers, Diluents & Binders
Excipient Details : KoVidone® K25 is used as a low viscosity wet binder in solid dosage forms such as capsules and tablets.
Pharmacopoeia Ref : USP/NF, EP, JP, KP, IP, BP
Technical Specs : NA
Ingredient(s) : Polyvinylpyrrolidone
Dosage Form : Injectable / Parenteral
Grade : Parenteral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Co-Processed Excipients
Excipient Details : DiCOM-DC S604 is used as a DC co-processed excipient with a combination of various polymers that act as fillers and binders in tablets and capsules.
Pharmacopoeia Ref : In-house
Technical Specs : Polyvinyl Pyrrolidone 97% & 93%
Ingredient(s) : Calcium Carbonate Excipient
Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Capsule
Grade : Oral
Application : Fillers, Diluents & Binders
Pharmacopoeia Ref : USP-NF, EP, BP, IP, JP, FCC
Technical Specs : PVP K-K-30/ K-17/ K19/ K25/ K90
Ingredient(s) : Povidone
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Brand Name : Microlex® PVD K30
Application : Solubilizers
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Also Available as Microlex® PVD K90.
Ingredient(s) : Povidone
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?