
Synopsis
Synopsis
0
JDMF
0
EU WC
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
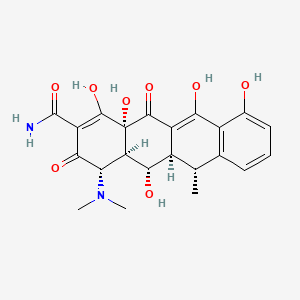

1. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4s-(4alpha,4aalpha,5alpha,5aalpha,6alpha,12aalpha))-
2. Alpha 6 Deoxyoxytetracycline
3. Alpha-6-deoxyoxytetracycline
4. Atridox
5. Bmy 28689
6. Bmy-28689
7. Bmy28689
8. Bu 3839t
9. Bu-3839t
10. Bu3839t
11. Doryx
12. Doxycycline Calcium
13. Doxycycline Calcium Salt (1:2)
14. Doxycycline Chinoin
15. Doxycycline Hemiethanolate
16. Doxycycline Hyclate
17. Doxycycline Monohydrate
18. Doxycycline Monohydrochloride, 6 Epimer
19. Doxycycline Monohydrochloride, 6-epimer
20. Doxycycline Monohydrochloride, Dihydrate
21. Doxycycline Phosphate (1:1)
22. Doxycycline-chinoin
23. Hydramycin
24. Oracea
25. Periostat
26. Vibra Tabs
27. Vibra-tabs
28. Vibramycin
29. Vibramycin Novum
30. Vibravenos
1. 564-25-0
2. Vibramycin
3. Doxytetracycline
4. Doxycycline (anhydrous)
5. Doxiciclina
6. Doxycyclinum
7. Monodox
8. Doxycycline Hyclate
9. Doxycycline Anhydrous
10. 6alpha-deoxy-5-oxytetracycline
11. 5-hydroxy-alpha-6-deoxytetracycline
12. Vibramycine
13. Doxychel
14. 6-alpha-deoxy-5-oxytetracycline
15. Doxycyclin
16. Alpha-6-deoxy-5-hydroxytetracycline
17. Bmy-28689
18. Anhydrous Doxycycline
19. Liviatin
20. Doxycycline (inn)
21. Doxy-caps
22. Deoxymykoin
23. Dossiciclina
24. Doxitard
25. Doxivetin
26. Doxycen
27. Investin
28. Chebi:50845
29. Doxy-puren
30. Alpha-doxycycline
31. Doxy-tabs
32. Alpha-6-deoxyoxytetracycline
33. Mmv000011
34. (4s,4ar,5s,5ar,6r,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide
35. Doxysol
36. Jenacyclin
37. 6-deoxyoxytetracycline
38. Dossiciclina [dcit]
39. Doxiciclina [italian]
40. Doxycycline [inn]
41. (4s,4ar,5s,5ar,6r,12as)-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
42. 2-naphthacenecarboxamide,4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4s,4ar,5s,5ar,6r,12as)-
43. Doxycyclinum [inn-latin]
44. Doxycycline (internal Use)
45. Doxiciclina [inn-spanish]
46. 334895s862
47. Bu-3839t
48. Zenavod
49. Doxcycline Anhydrous
50. Oxytetracycline, 6-deoxy-
51. Doxycycline (tn)
52. Doxychel (tn)
53. Vivox (*hyclate)
54. Dmsc (*fosfatex)
55. Nsc633557
56. Hsdb 3071
57. Monodox (*monohydrate)
58. Bmy28689
59. Bu 3839t
60. 24390-14-5
61. Einecs 209-271-1
62. Bmy 28689
63. Vibramycin (*monohydrate)
64. 4-epioxytetracycline, 6-deoxy-
65. Gs-3065 (*monohydrate)
66. Doxycycline [usan:inn:ban]
67. Sr-01000075844
68. Unii-334895s862
69. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4s-(4alpha,4aalpha,5alpha,5aalpha,6alpha,12aalpha))-
70. Ab08 (*fosfatex)
71. Spectrum_000807
72. (4s,4ar,5s,5ar,6r,12as)-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamidehydrochloride
73. Spectrum2_000143
74. Spectrum3_000408
75. Spectrum4_000527
76. Spectrum5_000947
77. Doxycycline [hsdb]
78. Upcmld-dp021
79. Vibramycin, Doxytetracycline
80. Chembl1433
81. Lopac0_000405
82. Schembl40930
83. Schembl66828
84. Bspbio_001936
85. Doxycycline [who-dd]
86. Kbiogr_001133
87. Kbioss_001287
88. Bidd:gt0146
89. Divk1c_000345
90. Spbio_000246
91. Chembl436921
92. Gtpl6464
93. Schembl1176275
94. Dtxsid0037653
95. Schembl17826665
96. Schembl19270298
97. Schembl21753474
98. Upcmld-dp021:001
99. Bcbcmap01_000024
100. Kbio1_000345
101. Kbio2_001287
102. Kbio2_003855
103. Kbio2_006423
104. Kbio3_001156
105. Dtxsid80992212
106. Ninds_000345
107. Doxycycline Anhydrous [mi]
108. Hms2090e06
109. Doxytetracycline; Doxycycline
110. Hy-n0565
111. Lmpk07000001
112. S5159
113. Zinc16052277
114. Anhydrous Doxycycline [mart.]
115. Akos015900372
116. Zinc100056779
117. Zinc100302137
118. Zinc100611210
119. Zinc109562088
120. Ccg-204498
121. Db00254
122. Sdccgsbi-0050391.p005
123. Idi1_000345
124. Smp1_000107
125. Ncgc00161602-01
126. Ncgc00161602-03
127. Ncgc00161602-04
128. Ncgc00161602-05
129. Ncgc00161602-07
130. Ncgc00161602-14
131. Ncgc00188979-01
132. (4s,4ar,5s,5ar,6r,12as)-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide
133. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4s,4ar,5s,5ar,6r,12as)
134. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4s,4ar,5s,5ar,6r,12as)-
135. 2-naphthacenecarboxamide, 4-alpha-s-(dimethylamino)-1,4,4a-alpha-5,5a-alpha,6,11,12a-octahydro-3,5-alpha,10,12,12a-alpha-pentahydroxy-6-alpha-methyl-1,11-dioxo-
136. As-13499
137. Sbi-0050391.p004
138. Cs-0009105
139. C06973
140. D07876
141. Ab00053465-03
142. Ab00053465_04
143. Ab00053465_05
144. 564d250
145. A817263
146. Doxycycline, Antibiotic For Culture Media Use Only
147. Q422442
148. Sr-01000075844-7
149. Vibramycin;doxytetracycline;doxiciclina;doxycyclinum
150. W-105517
151. Sr-01000075844-18
152. (4s,4ar,5s,5ar,6r,12as)-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide
153. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4s-(4.alpha.,4a.alpha.,5.alpha.,5a.alpha.,6.alpha.,12a.alpha.))-
154. 4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-3,4,4a,5,5a,6,12,12a-octahydrotetracene-2-carboximidic Acid
155. 7164-70-7
| Molecular Weight | 444.4 g/mol |
|---|---|
| Molecular Formula | C22H24N2O8 |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 2 |
| Exact Mass | 444.15326573 g/mol |
| Monoisotopic Mass | 444.15326573 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 956 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 18 | |
|---|---|
| Drug Name | Atridox |
| PubMed Health | Doxycycline (By mouth) |
| Drug Classes | Amebicide, Amebicide, Intestinal, Antiacne, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent |
| Drug Label | Doxycycline is a broad-spectrum antibacterial synthetically derived from oxytetracycline. Monodox 100 mg, 75 mg, and 50 mg capsules contain doxycycline monohydrate equivalent to 100 mg, 75 mg, or 50 mg of doxycycline for oral administration. The chem... |
| Active Ingredient | Doxycycline hyclate |
| Dosage Form | System, extended release |
| Route | Periodontal |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Tolmar |
| 2 of 18 | |
|---|---|
| Drug Name | Doryx |
| PubMed Health | Doxycycline (By mouth) |
| Drug Classes | Amebicide, Amebicide, Intestinal, Antiacne, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent |
| Active Ingredient | Doxycycline hyclate |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | eq 100mg base; eq 80mg base; eq 200mg base; eq 150mg base; eq 75mg base |
| Market Status | Prescription |
| Company | Mayne Pharma |
| 3 of 18 | |
|---|---|
| Drug Name | Doxy 100 |
| PubMed Health | Doxycycline (Subgingival) |
| Drug Classes | Antibacterial |
| Drug Label | DORYX Capsules contain specially coated pellets of doxycycline hyclate, a broad-spectrum antibiotic synthetically derived from oxytetracycline, in a delayed-release formulation for oral administration.The structural formula for doxycycline hyclate is... |
| Active Ingredient | Doxycycline hyclate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 100mg base/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 4 of 18 | |
|---|---|
| Drug Name | Doxycycline |
| PubMed Health | Doxycycline |
| Drug Classes | Amebicide, Amebicide, Intestinal, Antiacne, Antibacterial, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent |
| Drug Label | Doxycycline for Injection, USP is a sterile, lyophilized powder prepared from a solution of doxycycline hyclate, ascorbic acid and mannitol in Water for Injection. Doxycycline hyclate is a broad spectrum antibiotic derived from oxytetracycline. It is... |
| Active Ingredient | Doxycycline; Doxycycline hyclate |
| Dosage Form | Injectable; Tablet; Capsule; For suspension |
| Route | oral; Injection; Oral |
| Strength | eq 100mg base; eq 50mg base; eq 150mg base; 40mg; eq 25mg base/5ml; eq 75mg base; eq 100mg base/vial |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Ranbaxy; Lannett; Eurohlth Intl; Lupin; Agila Speclts; Chartwell Life Sci; Mylan; Par Pharm; Impax Labs; Heritage Pharms |
| 5 of 18 | |
|---|---|
| Drug Name | Doxycycline hyclate |
| PubMed Health | Doxycycline (Injection) |
| Drug Classes | Antibiotic, Antimalarial, Antiprotozoal |
| Drug Label | Doxycycline hyclate is a broad-spectrum antibiotic synthetically derived from oxytetracycline. The structural formula is as follows:with a molecular formula of C22H24N2O8H2O and a molecular weight of 462.46. The chemical designation for doxycycline i... |
| Active Ingredient | Doxycycline hyclate |
| Dosage Form | Tablet; Capsule, delayed release; Capsule; Tablet, delayed release |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base; eq 150mg base; eq 20mg base; eq 75mg base |
| Market Status | Prescription |
| Company | Corepharma; Mutual Pharma; Vintage Pharms; Mylan Pharms; Blu Caribe; Ivax Sub Teva Pharms; Actavis Elizabeth; Larken Labs; Lannett; Mutual Pharm; Actavis Labs Fl; Medicis; Chartwell Life Sci; Hikma Pharms; Mylan; Impax Labs; Heritage Pharms |
| 6 of 18 | |
|---|---|
| Drug Name | Monodox |
| Drug Label | Vibramycin is an antibacterial drug synthetically derived from oxytetracycline, and is available as Vibramycin Monohydrate (doxycycline monohydrate); Vibramycin Hyclate and Vibra-Tabs (doxycycline hydrochloride hemiethanolate hemihydrate); and Vibram... |
| Active Ingredient | Doxycycline |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base; eq 75mg base |
| Market Status | Prescription |
| Company | Aqua Pharms |
| 7 of 18 | |
|---|---|
| Drug Name | Oracea |
| PubMed Health | Doxycycline |
| Drug Classes | Amebicide, Amebicide, Intestinal, Antiacne, Antibacterial, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent |
| Drug Label | Doxycycline hyclate is a broad-spectrum antibiotic synthetically derived from oxytetracycline. The structural formula is as follows:with a molecular formula of C22H24N2O8H2O and a molecular weight of 462.46. The chemical designation for doxycycline i... |
| Active Ingredient | Doxycycline |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 40mg |
| Market Status | Prescription |
| Company | Galderma Labs |
| 8 of 18 | |
|---|---|
| Drug Name | Periostat |
| PubMed Health | Doxycycline |
| Drug Classes | Amebicide, Amebicide, Intestinal, Antiacne, Antibacterial, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent |
| Drug Label | ORACEA (doxycycline, USP) Capsules 40 mg are hard gelatin capsule shells filled with two types of doxycycline beads (30 mg immediate release and 10 mg delayed release) that together provide a dose of 40 mg of anhydrous doxycycline (C22H24N2O8).The st... |
| Active Ingredient | Doxycycline hyclate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 20mg base |
| Market Status | Prescription |
| Company | Galderma Labs |
| 9 of 18 | |
|---|---|
| Drug Name | Vibramycin |
| Active Ingredient | Doxycycline calcium; Doxycycline hyclate; Doxycycline |
| Dosage Form | Capsule; Suspension; For suspension |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base; eq 50mg base/5ml; eq 25mg base/5ml |
| Market Status | Prescription |
| Company | Pfizer |
| 10 of 18 | |
|---|---|
| Drug Name | Atridox |
| PubMed Health | Doxycycline (By mouth) |
| Drug Classes | Amebicide, Amebicide, Intestinal, Antiacne, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent |
| Drug Label | Doxycycline is a broad-spectrum antibacterial synthetically derived from oxytetracycline. Monodox 100 mg, 75 mg, and 50 mg capsules contain doxycycline monohydrate equivalent to 100 mg, 75 mg, or 50 mg of doxycycline for oral administration. The chem... |
| Active Ingredient | Doxycycline hyclate |
| Dosage Form | System, extended release |
| Route | Periodontal |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Tolmar |
| 11 of 18 | |
|---|---|
| Drug Name | Doryx |
| PubMed Health | Doxycycline (By mouth) |
| Drug Classes | Amebicide, Amebicide, Intestinal, Antiacne, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent |
| Active Ingredient | Doxycycline hyclate |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | eq 100mg base; eq 80mg base; eq 200mg base; eq 150mg base; eq 75mg base |
| Market Status | Prescription |
| Company | Mayne Pharma |
| 12 of 18 | |
|---|---|
| Drug Name | Doxy 100 |
| PubMed Health | Doxycycline (Subgingival) |
| Drug Classes | Antibacterial |
| Drug Label | DORYX Capsules contain specially coated pellets of doxycycline hyclate, a broad-spectrum antibiotic synthetically derived from oxytetracycline, in a delayed-release formulation for oral administration.The structural formula for doxycycline hyclate is... |
| Active Ingredient | Doxycycline hyclate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 100mg base/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 13 of 18 | |
|---|---|
| Drug Name | Doxycycline |
| PubMed Health | Doxycycline |
| Drug Classes | Amebicide, Amebicide, Intestinal, Antiacne, Antibacterial, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent |
| Drug Label | Doxycycline for Injection, USP is a sterile, lyophilized powder prepared from a solution of doxycycline hyclate, ascorbic acid and mannitol in Water for Injection. Doxycycline hyclate is a broad spectrum antibiotic derived from oxytetracycline. It is... |
| Active Ingredient | Doxycycline; Doxycycline hyclate |
| Dosage Form | Injectable; Tablet; Capsule; For suspension |
| Route | oral; Injection; Oral |
| Strength | eq 100mg base; eq 50mg base; eq 150mg base; 40mg; eq 25mg base/5ml; eq 75mg base; eq 100mg base/vial |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Ranbaxy; Lannett; Eurohlth Intl; Lupin; Agila Speclts; Chartwell Life Sci; Mylan; Par Pharm; Impax Labs; Heritage Pharms |
| 14 of 18 | |
|---|---|
| Drug Name | Doxycycline hyclate |
| PubMed Health | Doxycycline (Injection) |
| Drug Classes | Antibiotic, Antimalarial, Antiprotozoal |
| Drug Label | Doxycycline hyclate is a broad-spectrum antibiotic synthetically derived from oxytetracycline. The structural formula is as follows:with a molecular formula of C22H24N2O8H2O and a molecular weight of 462.46. The chemical designation for doxycycline i... |
| Active Ingredient | Doxycycline hyclate |
| Dosage Form | Tablet; Capsule, delayed release; Capsule; Tablet, delayed release |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base; eq 150mg base; eq 20mg base; eq 75mg base |
| Market Status | Prescription |
| Company | Corepharma; Mutual Pharma; Vintage Pharms; Mylan Pharms; Blu Caribe; Ivax Sub Teva Pharms; Actavis Elizabeth; Larken Labs; Lannett; Mutual Pharm; Actavis Labs Fl; Medicis; Chartwell Life Sci; Hikma Pharms; Mylan; Impax Labs; Heritage Pharms |
| 15 of 18 | |
|---|---|
| Drug Name | Monodox |
| Drug Label | Vibramycin is an antibacterial drug synthetically derived from oxytetracycline, and is available as Vibramycin Monohydrate (doxycycline monohydrate); Vibramycin Hyclate and Vibra-Tabs (doxycycline hydrochloride hemiethanolate hemihydrate); and Vibram... |
| Active Ingredient | Doxycycline |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base; eq 75mg base |
| Market Status | Prescription |
| Company | Aqua Pharms |
| 16 of 18 | |
|---|---|
| Drug Name | Oracea |
| PubMed Health | Doxycycline |
| Drug Classes | Amebicide, Amebicide, Intestinal, Antiacne, Antibacterial, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent |
| Drug Label | Doxycycline hyclate is a broad-spectrum antibiotic synthetically derived from oxytetracycline. The structural formula is as follows:with a molecular formula of C22H24N2O8H2O and a molecular weight of 462.46. The chemical designation for doxycycline i... |
| Active Ingredient | Doxycycline |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 40mg |
| Market Status | Prescription |
| Company | Galderma Labs |
| 17 of 18 | |
|---|---|
| Drug Name | Periostat |
| PubMed Health | Doxycycline |
| Drug Classes | Amebicide, Amebicide, Intestinal, Antiacne, Antibacterial, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent |
| Drug Label | ORACEA (doxycycline, USP) Capsules 40 mg are hard gelatin capsule shells filled with two types of doxycycline beads (30 mg immediate release and 10 mg delayed release) that together provide a dose of 40 mg of anhydrous doxycycline (C22H24N2O8).The st... |
| Active Ingredient | Doxycycline hyclate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 20mg base |
| Market Status | Prescription |
| Company | Galderma Labs |
| 18 of 18 | |
|---|---|
| Drug Name | Vibramycin |
| Active Ingredient | Doxycycline calcium; Doxycycline hyclate; Doxycycline |
| Dosage Form | Capsule; Suspension; For suspension |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base; eq 50mg base/5ml; eq 25mg base/5ml |
| Market Status | Prescription |
| Company | Pfizer |
Antibiotics, Tetracycline
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
AGAINST GRAM-POSITIVE BACTERIA IT IS ABOUT TWICE AS POTENT AS TETRACYCLINE, EXCEPT THAT IT IS UP TO 10 TIMES AS POTENT AGAINST STREP VIRIDANS. FURTHERMORE, STRAINS OF STREP FAECALIS THAT ARE RESISTANT TO OTHER TETRACYCLINES MAY BE SENSITIVE TO DOXYCYCLINE.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1140
DOSE OF DOXYCYCLINE FOR ADULTS IS 100 MG EVERY 12 HR DURING FIRST 24 HR, FOLLOWED BY 100 MG ONCE DAY, OR TWICE DAILY WHEN SEVERE INFECTION IS PRESENT. CHILDREN OVER 8 YR SHOULD RECEIVE 4-5 MG/KG/DAY, DIVIDED INTO 2 EQUAL DOSES GIVEN @ 12 HR INTERVAL DURING FIRST DAY, AFTER WHICH SINGLE DOSE OF HALF THIS AMT IS ADMIN.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1120
AFFINITY OF DOXYCYCLINE FOR METALLIC IONS MAY NOT BE AS GREAT AS THAT OF OTHER TETRACYCLINES SINCE IT CAN BE GIVEN WITH FOOD OR MILK WITHOUT SIGNIFICANT INACTIVATION OR IMPAIRMENT OF ABSORPTION.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 746
For more Therapeutic Uses (Complete) data for DOXYCYCLINE (27 total), please visit the HSDB record page.
TETRACYCLINES SHOULD NOT BE ADMIN TO PREGNANT OR NURSING WOMEN & CHILDREN UNDER 8 YR UNLESS THERE ARE COMPELLING REASONS TO DO SO.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 1298
TREATMENT OF PREGNANT PATIENTS ... MAY PRODUCE DISCOLORATION OF TEETH IN THEIR OFFSPRING. ... CHILDREN UP TO 8 YR OLD MAY BE SUSCEPTIBLE ... TETRACYCLINES ARE DEPOSITED IN SKELETON DURING GESTATION. ... 40% DEPRESSION OF BONE GROWTH ... DEMONSTRATED IN PREMATURE INFANTS TREATED WITH THESE AGENTS. /TETRACYCLINES/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1122
TETRACYCLINES POSE SPECIAL DANGER IN PREGNANT WOMEN, WITH RESPECT TO POSSIBLE HEPATIC INJURY, PARTICULARLY IF USED FOR TREATMENT OF PYELONEPHRITIS, RELATIVELY COMMON OCCURRENCE IN SUCH PATIENTS INDEED, FATALITIES HAVE OCCURRED. /TETRACYCLINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1188
IT SHOULD BE EMPHASIZED THAT CROSS-SENSITIZATION AMONG VARIOUS TETRACYCLINES IS COMMON.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1122
For more Drug Warnings (Complete) data for DOXYCYCLINE (12 total), please visit the HSDB record page.
Doxycycline is indicated for the treatment of various infections by gram-positive and gram-negative bacteria, aerobes and anaerobes, as well other types of bacteria. A complete list of organisms is available in the FDA label and in the "indications" section of this drug entry. The following are some of the major infections that may be treated with doxycycline: Rocky mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsialpox, and tick fevers caused by Rickettsiae Respiratory tract infections caused by Mycoplasma pneumoniae Lymphogranuloma venereum caused by Chlamydia trachomatis Psittacosis (ornithosis) caused by Chlamydia psittaci Trachoma caused by Chlamydia trachomatis, although the infectious agent is not always eliminated as judged by immunofluorescence Inclusion conjunctivitis caused by Chlamydia trachomatis Uncomplicated urethral, endocervical or rectal infections in adults caused by Chlamydia trachomatis Nongonococcal urethritis caused by Ureaplasma urealyticum Relapsing fever due to Borrelia recurrentis **A note regarding anti-microbial resistance** It is important to note that doxycycline is not the drug of choice in the treatment of any type of staphylococcal infection. Up to 44 percent of strains of Streptococcus pyogenes and 74 percent of Streptococcus faecalis have been found to be resistant to tetracyclines. Therefore, tetracyclines such as doxycycline should not be used to treat streptococcal infections unless the microorganism has been demonstrated to be susceptible.
FDA Label
Treatment of periodontal disease in dogs.
Periodontal pocket probing depths > =4 mm are evidence of disease that may be responsive to treatment with the Doxirobe Gel. Use of this product as directed should result in attachment level gains, periodontal pocket depth reductions, local antimicrobial effect and improved gingival health. Noticeable improvements in these parameters should be evident within 2-4 weeks following treatment. The response in individual dogs is dependent on the severity of the condition and rigor of adjunctive therapy.
The tetracyclines, including doxycycline, are mainly bacteriostatic and are thought to exert antimicrobial effects by the inhibition of protein synthesis. Bacteriostatic antibiotics suppress the growth of bacteria, or keep them in the stationary phase of growth. The tetracyclines, including doxycycline, have a similar antimicrobial spectrum of activity against a variety of gram-positive and gram-negative microorganisms, treating numerous infectious diseases. Cross-resistance of these microorganisms to tetracyclines is a common occurrence. Doxycycline shows favorable intra-cellular penetration, with bacteriostatic activity on a wide range of bacteria. Doxycycline has antiparasitic effects,,. In addition to the above effects, this drug has demonstrated anti-inflammatory actions, which may help to manage inflammatory conditions such as rosacea.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
QJ01AA02
J01AA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AB - Antiinfectives and antiseptics for local oral treatment
A01AB22 - Doxycycline
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01A - Tetracyclines
J01AA - Tetracyclines
J01AA02 - Doxycycline
Absorption
Tetracyclines, such as doxycycline, are readily absorbed and are bound to plasma proteins by varying degrees. Doxycycline is almost completely absorbed after oral administration. This drug is highly lipid soluble and has a low affinity for calcium binding. Absorption is not significantly affected by the concomitant ingestion of food or milk. Peak serum levels of approximately 2.6 mcg/ml are reached at 2 hours following a 200 mg tablet oral dose.
Route of Elimination
Mainly the urine and feces as active and unchanged drug. Between 40% and 60% of an administered dose can be accounted for in the urine by 92 hours, and approximately 30% can be accounted for in the feces.
Volume of Distribution
Doxycycline diffuses readily into most body tissues, fluid and/or cavities and the volume of distribution has been measured as 0.7 L/kg.
Clearance
The excretion of doxycycline by the kidney is about 40% over 72 hours in individuals with normal kidney function (creatinine clearance approximately 75 mL/min). This rate may fall as low as 1-5% over 72 hours in individuals with severe renal insufficiency (creatinine clearance below 10 mL/min). Some clinical studies have shown no major difference in serum half-life of doxycycline (range 18-22 hours) in patients with normal and severely impaired renal function. Hemodialysis does not affect serum half-life of doxycycline.
RELATIVE INSENSITIVITY OF DOXYCYCLINE PHARMACOKINETICS TO RENAL INSUFFICIENCY HAS ... BEEN DEMONSTRATED IN HUMANS & APPEARS TO BE ASSOC WITH INCR FECAL EXCRETION OWING TO DIFFUSION OF DRUG INTO LUMEN OF SMALL INTESTINE. ... RENAL CLEARANCE OF ACTIVE ANTIBIOTIC IS ... 20 ... ML/MIN FOR DOXYCYCLINE ... .
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 174
SERUM LEVELS OF DOXYCYCLINE ARE EQUIV WHETHER DRUG IS DOSED BY IV OR PER OS ROUTE. AFTER MULTIPLE DAILY IV DOSES OF 200 MG, SERUM LEVELS ... FLUCTUATED BETWEEN 5-6 & 1-2 UG/ML, WHICH IS ABOVE MIN INHIBITORY CONCN FOR MOST SUSCEPTIBLE PATHOGENS.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 174
URINARY EXCRETION OF ... DOXYCYCLINE IS INCR @ HIGH URINARY PH VALUES. ALKALINE TREATMENT OF SUBJECTS RESULTED IN 24% INCR IN CUMULATIVE URINARY TETRACYCLINE EXCRETION COMPARED WITH ACID TREATMENT (P< 0.05) & 54% INCR FOR DOXYCYCLINE (P LESS THAN 0.05). RENAL CLEARANCE ... INCR DURING ALKALINE TREATMENT ... .
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 173
... MORE COMPLETELY ABSORBED AFTER ORAL ADMIN THAN OTHER TETRACYCLINES ... IN PLASMA, IT IS ABOUT 90% PROTEIN BOUND, WHICH IS HIGHEST DEGREE FOR ANY OF TETRACYCLINES.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1140
For more Absorption, Distribution and Excretion (Complete) data for DOXYCYCLINE (20 total), please visit the HSDB record page.
Doxycycline is metabolized in the liver and gastrointestinal tract and concentrated in bile,. Major metabolic pathways of doxycycline have not been identified, however, enzyme inducers have been found to decrease the half-life of doxycycline.
/DOXYCYCLINE/ IS EXCRETED IN FECES (UP TO 90%) AS INACTIVE CONJUGATE OR PERHAPS AS CHELATE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1186
Although it was previously suggested that doxycycline is partially metabolized in the liver, recent studies indicate that the drug is not metabolized but is partially deactivated in the intestine by chelate formation.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 319
16.33 hr ( 4.53 sd).
DOXYCYCLINE: ROUTES OF EXCRETION: HEPATIC, RENAL; NORMAL HALF-LIFE: 20 HR; MAINTENANCE DOSE INTERVALS: 12-24 HR.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1109
DOXYCYCLINE IS LONG-ACTING, HAVING SERUM HALF-LIFE OF 15-17 HR AFTER INITIAL DOSE & ABOUT 22 HR AFTER 4TH DAY OF TREATMENT.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 736
The serum half-life of doxycycline is 14-17 hr after a single dose and 22-24 hr after multiple doses in patients with normal renal function. In patients with severe renal impairment, the serum half-life of doxycycline is reported to be 18-26 hr after a single dose, and 20-30 hr after multiple doses. It appears that serum half-life of doxycycline is not altered in patients undergoing hemodialysis. In patients with normal renal function, approximately 20-26% of a single oral or iv dose of doxycycline is excreted in urine and 20-40% is excreted in feces within 48 hr as active drug. In patients with creatinine clearances less than 10 ml/minute, the fraction of doxycycline excreted in urine within 72 hr may decrease to about 1-5%.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 319
In bacterial replication, an interaction that is important for translation initiation of proteins occurs at the 3 end of the 16S rRNA, found on the ribosome on the 30S subunit,,. The 30S subunit is the smaller subunit of the ribosome of prokaryotes, including bacteria. Tetracyclines such as doxycycline are thought to inhibit translation by binding to the 16S rRNA portion of the ribosome, preventing binding of tRNA to the RNA-30S bacterial ribosomal subunit, which is necessary for the delivery of amino acids for protein synthesis. As a result of the above actions, the initiation of protein synthesis by polyribosome formation is blocked. This stops the replication of bacteria and produces a bacteriostatic effect.
TETRACYCLINES INHIBIT BACTERIAL PROTEIN SYNTHESIS. ... ONCE TETRACYCLINES GAIN ACCESS TO THE BACTERIAL CELL, THEY BIND PRINCIPALLY TO 30 S SUBUNITS OF BACTERIAL RIBOSOMES. THEY APPEAR TO PREVENT ACCESS OF AMINOACYL T-RNA TO M-RNA-RIBOSOME COMPLEX. ... ONLY SMALL PORTION OF DRUG IS IRREVERSIBLY BOUND, & INHIBITORY EFFECTS OF THE TETRACYCLINESARE REVERSIBLE WHEN THE DRUG IS REMOVED. /TETRACYCLINES/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1118
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13944
Submission : 1999-01-13
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2021-08-10
Pay. Date : 2021-08-06
DMF Number : 25062
Submission : 2011-06-16
Status : Active
Type : II

NDC Package Code : 47621-017
Start Marketing Date : 2021-01-27
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-05-26
Pay. Date : 2014-05-14
DMF Number : 18959
Submission : 2005-11-09
Status : Active
Type : II

NDC Package Code : 55488-0200
Start Marketing Date : 1981-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2024-04-16
Pay. Date : 2024-04-02
DMF Number : 39292
Submission : 2023-12-22
Status : Active
Type : II

NDC Package Code : 55488-0200
Start Marketing Date : 1981-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2013-01-11
Pay. Date : 2012-12-11
DMF Number : 13710
Submission : 1998-09-01
Status : Active
Type : II

Certificate Number : R1-CEP 1997-115 - Rev 07
Issue Date : 2021-09-10
Type : Chemical
Substance Number : 820
Status : Valid

NDC Package Code : 50909-8106
Start Marketing Date : 2014-07-30
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2013-01-09
Pay. Date : 2012-12-11
DMF Number : 23638
Submission : 2010-03-18
Status : Active
Type : II

NDC Package Code : 50909-8106
Start Marketing Date : 2014-07-30
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13500
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13040
Submission : 1998-06-25
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13500
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-05-26
Pay. Date : 2014-05-14
DMF Number : 18959
Submission : 2005-11-09
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-01-09
Pay. Date : 2012-12-11
DMF Number : 23638
Submission : 2010-03-18
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-01-11
Pay. Date : 2012-12-11
DMF Number : 13710
Submission : 1998-09-01
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18313
Submission : 2005-04-26
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13040
Submission : 1998-06-25
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2021-08-10
Pay. Date : 2021-08-06
DMF Number : 25062
Submission : 2011-06-16
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 14295
Submission : 1999-07-14
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13944
Submission : 1999-01-13
Status : Active
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13450
Submission : 1998-09-01
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2004-162 - Rev 00
Status : Withdrawn by Holder
Issue Date : 2011-06-01
Type : Chemical
Substance Number : 820

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1997-115 - Rev 07
Status : Valid
Issue Date : 2021-09-10
Type : Chemical
Substance Number : 820

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2012-243 - Rev 00
Status : Valid
Issue Date : 2019-01-28
Type : Chemical
Substance Number : 820

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2003-226 - Rev 00
Status : Withdrawn by EDQM Failure to CEP pro...
Issue Date : 2005-04-27
Type : Chemical
Substance Number : 820

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1997-072 - Rev 03
Status : Withdrawn by Holder
Issue Date : 2008-03-17
Type : Chemical
Substance Number : 820

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1996-064 - Rev 04
Status : Withdrawn by EDQM Failure to CEP pro...
Issue Date : 2007-06-01
Type : Chemical
Substance Number : 820

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 1999-157 - Rev 05
Status : Valid
Issue Date : 2024-03-06
Type : Chemical
Substance Number : 820

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2013-052 - Rev 00
Status : Valid
Issue Date : 2019-02-12
Type : Chemical
Substance Number : 820

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]  Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
About the Company : Established in 1994, Rochem is a distributor of pharmaceutical, food, nutritional and animal health ingredients to some of the largest companies in the world. It sources high-quali...
About the Company : DKSH, founded with the goal of improving people's lives, assists businesses with market expansion and business growth in both existing and emerging markets. It has been fostering g...
About the Company : Changzhou Pharmaceutical Factory (CPF) is a subsidiary company of Shanghai Pharma Holdings Co., Ltd., It is headquartered in Changzhou, Jiangsu Province, China. The company was fou...

About the Company : At Freemen Nutra, the focus is on empowering both individuals and customers to achieve greater success. The company specializes in providing high-quality nutritional ingredients an...

About the Company : Hovione is an international company with over 60 years of experience as a Contract Development and Manufacturing Organization (CDMO) with a fully integrated offering of services fo...

About the Company : Huvepharma® Italia is a fast-growing global pharmaceutical company duly organized for developing, manufacturing and marketing advanced intermediates and active pharmaceutical ingr...

About the Company : Maithri Drugs is one of India's fast-growing pharmaceutical companies. Maithri's strategic focus is on active pharma ingredients (APIs). The company is widely recognized for its ex...

About the Company : NEMLabs is a leading developer & producer of quality affordable drugs, ointments and injectables across a variety of therapies.

About the Company : Osmopharm S.A. is a GMP approved pharmaceutical company located in Switzerland and specialized in development and production of modified release solid oral form drugs under contrac...

About the Company : Otto Brandes Gmbh was founded in 1923 and is an independent distributor of high quality Pharmaceutical Active Ingredients and excipients for the pharmaceutical industry. We regard ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?