
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
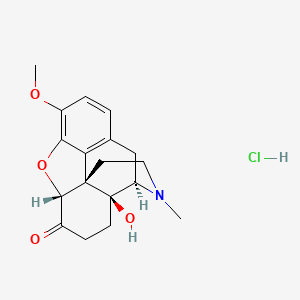

1. Dihydrohydroxycodeinone
2. Dihydrone
3. Dinarkon
4. Eucodal
5. Oxiconum
6. Oxycodeinon
7. Oxycodone
8. Oxycone
9. Oxycontin
10. Pancodine
11. Theocodin
1. 124-90-3
2. Oxycodone Hcl
3. Oxecta
4. Oxycontin
5. Endocodone
6. Roxicodone
7. Oxycodone Hydrochloride Cii
8. Chebi:7859
9. 4,5alpha-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one Hydrochloride
10. (4r,4as,7ar,12bs)-4a-hydroxy-9-methoxy-3-methyl-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one;hydrochloride
11. (4r,4as,7ar,12bs)-4a-hydroxy-9-methoxy-3-methyl-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one;hydron;chloride
12. Roxicodone (tn)
13. Oxycontin (tn)
14. (4r,4as,7ar,12bs)-4a-hydroxy-9-methoxy-3-methyl-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one;hydrochloride
15. Oxecta (tn)
16. Roxybond
17. Oxycodone Monohydrochloride
18. Schembl30095
19. Oxycodone Hydrochloride (usp)
20. Chembl1200890
21. Dtxsid80924674
22. Oxycodone Hydrochloride [mi]
23. Oxycodone Hydrochloride [usan]
24. Akos024457896
25. Oxycodone Hydrochloride [mart.]
26. Oxycodone Hydrochloride [vandf]
27. Anhydrous Oxycodone Hydrochloride
28. Oxycodone Hydrochloride [who-dd]
29. Oxycodone Hydrochloride, Analytical Standard
30. Oxycodone Hydrochloride [orange Book]
31. Oxycodone Hydrochloride Cii [usp-rs]
32. Oxycodone Hydrochloride [ep Monograph]
33. Tylox Component Oxycodone Hydrochloride
34. C08026
35. Codoxy Component Oxycodone Hydrochloride
36. D00847
37. Oxycet Component Oxycodone Hydrochloride
38. Oxycodone Hydrochloride [usp Monograph]
39. Roxicet Component Oxycodone Hydrochloride
40. Roxilox Component Oxycodone Hydrochloride
41. Troxyca Component Oxycodone Hydrochloride
42. Combunox Component Oxycodone Hydrochloride
43. Oxycodone Hydrochloride Component Of Tylox
44. Percocet Component Oxycodone Hydrochloride
45. Percodan Component Oxycodone Hydrochloride
46. Roxiprin Component Oxycodone Hydrochloride
47. Targiniq Component Oxycodone Hydrochloride
48. Xartemis Component Oxycodone Hydrochloride
49. Oxycodone Hydrochloride Component Of Codoxy
50. Oxycodone Hydrochloride Component Of Oxycet
51. Oxycodone Hydrochloride Component Of Roxicet
52. Oxycodone Hydrochloride Component Of Roxilox
53. Oxycodone Hydrochloride Component Of Troxyca
54. Oxycodone Hydrochloride Component Of Combunox
55. Oxycodone Hydrochloride Component Of Percocet
56. Oxycodone Hydrochloride Component Of Percodan
57. Oxycodone Hydrochloride Component Of Roxiprin
58. Oxycodone Hydrochloride Component Of Targiniq
59. Oxycodone Hydrochloride Component Of Xartemis
60. Percodan-demi Component Oxycodone Hydrochloride
61. Q27107601
62. Oxycodone Hydrochloride Component Of Percodan-demi
63. 14-hydroxy-3-methoxy-17-methyl-4,5alpha-epoxymorphinan-6-one Hydrochloride
64. Oxycodone Hydrochloride, European Pharmacopoeia (ep) Reference Standard
65. Oxycodone Hydrochloride, United States Pharmacopeia (usp) Reference Standard
66. (5?)-4,5-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one Hydrochloride
67. (5alpha,17s)-14-hydroxy-3-methoxy-17-methyl-6-oxo-4,5-epoxymorphinan-17-ium Chloride
68. 4,5.alpha.-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one Hydrochloride
69. Morphinan-6-one, 4,5-epoxy-14-hydroxy-3-methoxy-17-methyl-, Hydrochloride, (5.alpha.)-
70. Oxycodone Hydrochloride Solution, 1.0 Mg/ml In Methanol, Analytical Standard, For Drug Analysis
| Molecular Weight | 351.8 g/mol |
|---|---|
| Molecular Formula | C18H22ClNO4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 351.1237359 g/mol |
| Monoisotopic Mass | 351.1237359 g/mol |
| Topological Polar Surface Area | 59 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 553 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Oxecta |
| Drug Label | OXECTA (oxycodone HCl, USP) tablets are an immediate-release opioid analgesic intended for oral administration only. OXECTA contains oxycodone HCl, USP as the active analgesic ingredient. The tablets are round, convex, white and debossed with the str... |
| Active Ingredient | Oxycodone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 7.5mg; 5mg |
| Market Status | Prescription |
| Company | Acura Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Oxycodone hydrochloride |
| Drug Label | DESCRIPTIONOxyContin (oxycodone hydrochloride controlled-release) Tablets are an opioid analgesic supplied in 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 60 mg, 80 mg, and 160 mg tablet strengths for oral administration... |
| Active Ingredient | Oxycodone hydrochloride |
| Dosage Form | Tablet, extended release; Tablet; Capsule; Solution |
| Route | oral; Oral |
| Strength | 30mg; 100mg/5ml; 15mg; 5mg; 10mg; 5mg/5ml; 80mg; 40mg; 20mg |
| Market Status | Tentative Approval; Prescription |
| Company | Corepharma; Vintage Pharms; Amneal Pharms; Impax Pharms; Aurolife Pharma; Avanthi; Teva; Endo Pharms; Sun Pharm Inds; Coastal Pharms; Rhodes Pharms; Mallinckrodt; Roxane; Lannett Holdings; Nesher Pharms; Vistapharm; Actavis Elizabeth; Lehigh Valley; Alvog |
| 3 of 4 | |
|---|---|
| Drug Name | Oxecta |
| Drug Label | OXECTA (oxycodone HCl, USP) tablets are an immediate-release opioid analgesic intended for oral administration only. OXECTA contains oxycodone HCl, USP as the active analgesic ingredient. The tablets are round, convex, white and debossed with the str... |
| Active Ingredient | Oxycodone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 7.5mg; 5mg |
| Market Status | Prescription |
| Company | Acura Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Oxycodone hydrochloride |
| Drug Label | DESCRIPTIONOxyContin (oxycodone hydrochloride controlled-release) Tablets are an opioid analgesic supplied in 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 60 mg, 80 mg, and 160 mg tablet strengths for oral administration... |
| Active Ingredient | Oxycodone hydrochloride |
| Dosage Form | Tablet, extended release; Tablet; Capsule; Solution |
| Route | oral; Oral |
| Strength | 30mg; 100mg/5ml; 15mg; 5mg; 10mg; 5mg/5ml; 80mg; 40mg; 20mg |
| Market Status | Tentative Approval; Prescription |
| Company | Corepharma; Vintage Pharms; Amneal Pharms; Impax Pharms; Aurolife Pharma; Avanthi; Teva; Endo Pharms; Sun Pharm Inds; Coastal Pharms; Rhodes Pharms; Mallinckrodt; Roxane; Lannett Holdings; Nesher Pharms; Vistapharm; Actavis Elizabeth; Lehigh Valley; Alvog |
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?