
Synopsis
Synopsis
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Agon
2. Felo Biochemie
3. Felo Puren
4. Felo-puren
5. Felobeta
6. Felocor
7. Felodipin 1a Pharma
8. Felodipin Abz
9. Felodipin Al
10. Felodipin Azu
11. Felodipin Dura
12. Felodipin Heumann
13. Felodipin Ratiopharm
14. Felodipin Stada
15. Felodipin Von Ct
16. Felodipin-ratiopharm
17. Felodur
18. Felogamma
19. Fensel
20. Flodil
21. H 154 82
22. H 154-82
23. H 15482
24. Heumann, Felodipin
25. Modip
26. Munobal
27. Perfudal
28. Plendil
29. Renedil
30. Von Ct, Felodipin
1. 72509-76-3
2. Plendil
3. Flodil
4. Renedil
5. Feloday
6. Munobal
7. Splendil
8. Dl-felodipine
9. Perfudal
10. Prevex
11. Hydac
12. Modip
13. Agon
14. Felodipina
15. Felodipinum
16. Felodipinum [inn-latin]
17. 3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
18. Felodipina [inn-spanish]
19. H 154/82
20. Felodipine (plendil)
21. C08ca02
22. Cgh-869
23. Plendil Er
24. 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic Acid Ethyl Methyl Ester
25. 5-o-ethyl 3-o-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
26. Felogard
27. Mfcd00868316
28. Penedil
29. Preslow
30. Munobal Retard
31. Nsc-760343
32. Plendil Retard
33. H-154/82
34. Plendil Depottab
35. (+-)-ethyl Methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
36. Mls000069629
37. Ol961r6o2c
38. Chebi:585948
39. Felodur Er
40. Agon Sr
41. 3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydro-3,5-pyridinedicarboxylate
42. Ethyl Methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
43. Ncgc00015455-03
44. Plandil
45. Smr000058204
46. C18h19cl2no4
47. Dsstox_cid_3042
48. Dsstox_rid_76848
49. Dsstox_gsid_23042
50. Felodipine [usan:ban:inn]
51. Logimax
52. Ethyl Methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
53. (+/-)-ethyl Methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
54. (rs)-3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
55. Plendil (tn)
56. Sr-01000075890
57. Brn 4331472
58. Unii-ol961r6o2c
59. Perfuda
60. Plendil;renedil
61. Felodipine, Solid
62. Felodipine,(s)
63. Felodipine [usan:usp:inn:ban]
64. 3,5-dicarboxylate
65. Felodipine- Bio-x
66. Prestwick_797
67. Cas-72509-76-3
68. 3,5-pyridinedicarboxylic Acid, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, Ethyl Methyl Ester
69. Felodipine (dl Form)
70. Felodipine [mi]
71. Felodipine [inn]
72. Felodipine [jan]
73. Opera_id_1873
74. Prestwick0_000478
75. Prestwick1_000478
76. Prestwick2_000478
77. Prestwick3_000478
78. (.+/-.)-felodipine
79. Felodipine [usan]
80. Felodipine [vandf]
81. F 9677
82. Felodipine [mart.]
83. 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinecarboxylic Acid Ethyl Methyl Ester
84. Chembl1480
85. Felodipine [usp-rs]
86. Felodipine [who-dd]
87. Lopac0_000508
88. Schembl26398
89. Bspbio_000616
90. Ae-641/11429675
91. Mls001077361
92. Mls001333735
93. Mls002153409
94. Mls002153832
95. Mls003876820
96. Bidd:gt0733
97. Spbio_002555
98. Bpbio1_000678
99. Felodipine (jp17/usp/inn)
100. Gtpl4190
101. (r)-(+)-felodipine-[d5]
102. Chembl3196476
103. Dtxsid4023042
104. Felodipine [orange Book]
105. Schembl13460298
106. Felodipine [ep Monograph]
107. Felodipine [usp Impurity]
108. Lexxel Component Felodipine
109. Bdbm189379
110. Felodipine [usp Monograph]
111. Hms1569o18
112. Hms2089j05
113. Hms2096o18
114. Hms2232d24
115. Hms3259f12
116. Hms3261f17
117. Hms3651o21
118. Hms3713o18
119. Hms3884i14
120. Pharmakon1600-01505887
121. Bcp02192
122. Bcp22685
123. Hy-b0309
124. 2,6-dimethyl-1,4-dihydropyridine-
125. Tox21_110155
126. Tox21_500508
127. Ca-236
128. Nsc760343
129. S1885
130. Felodipine Component Of Lexxel
131. Akos015891545
132. Felodipine 100 Microg/ml In Methanol
133. Tox21_110155_1
134. Ac-2124
135. Bcp9000680
136. Ccg-204599
137. Db01023
138. Ks-1264
139. Lp00508
140. Nc00721
141. Nsc 760343
142. Sdccgsbi-0050492.p002
143. O5-ethyl O3-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
144. Ncgc00015455-04
145. Ncgc00015455-05
146. Ncgc00015455-06
147. Ncgc00015455-07
148. Ncgc00015455-08
149. Ncgc00015455-10
150. Ncgc00015455-24
151. Ncgc00024087-02
152. Ncgc00093906-01
153. Ncgc00093906-02
154. Ncgc00261193-01
155. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-4-(2,3-dichlorophenyl)-2,6-dimethyl-, Ethyl Methyl Ester
156. 3,5-pyridinedicarboxylic Acid, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, Ethyl Methyl Ester, (+-)-
157. 4-(2,3-dichloro-phenyl)-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic Acid 3-ethyl Ester 5-methyl Ester
158. Ac-24403
159. Bf164445
160. Felodipine 100 Microg/ml In Acetonitrile
161. Smr002529504
162. Sy053174
163. 3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-
164. Eu-0100508
165. F0814
166. Ft-0626393
167. Ft-0660933
168. Ft-0668475
169. Sw219299-1
170. En300-70726
171. D00319
172. 509f763
173. Q420644
174. Sr-01000075890-1
175. Sr-01000075890-4
176. Brd-a30815329-001-03-0
177. Z239864852
178. Felodipine, European Pharmacopoeia (ep) Reference Standard
179. Felodipine, United States Pharmacopeia (usp) Reference Standard
180. 3-ethyl5-methyl4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
181. (.+/-.) Ethyl Methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
182. 3,5-pyridinedicarboxylic Acid 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, Ethyl Methyl Ester, (+/-)-
183. 3,5-pyridinedicarboxylic Acid, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, 3-ethyl-5-methylester
184. 3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydro-3,5-pyridinedicarboxylate #
| Molecular Weight | 384.2 g/mol |
|---|---|
| Molecular Formula | C18H19Cl2NO4 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 383.0691135 g/mol |
| Monoisotopic Mass | 383.0691135 g/mol |
| Topological Polar Surface Area | 64.6 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 614 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Felodipine |
| PubMed Health | Felodipine (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
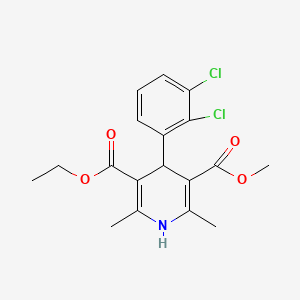
| Drug Label | Felodipine is a calcium antagonist (calcium channel blocker). Felodipine is a dihydropyridine derivative that is chemically described as ethyl methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2, 6-dimethyl-3,5-pyridinedicarboxylate. Its molecular formula... |
| Active Ingredient | Felodipine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 2.5mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Wockhardt; Glenmark Generics; Mutual Pharm; Aurobindo Pharma; Torrent Pharms; Ranbaxy Labs; Mylan; Heritage Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Plendil |
| PubMed Health | Felodipine (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | PLENDIL (felodipine) is a calcium antagonist (calcium channel blocker). Felodipine is a dihydropyridine derivative that is chemically described as ethyl methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate. Its empirica... |
| Active Ingredient | Felodipine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 2.5mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Astrazeneca |
| 3 of 4 | |
|---|---|
| Drug Name | Felodipine |
| PubMed Health | Felodipine (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | Felodipine is a calcium antagonist (calcium channel blocker). Felodipine is a dihydropyridine derivative that is chemically described as ethyl methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2, 6-dimethyl-3,5-pyridinedicarboxylate. Its molecular formula... |
| Active Ingredient | Felodipine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 2.5mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Wockhardt; Glenmark Generics; Mutual Pharm; Aurobindo Pharma; Torrent Pharms; Ranbaxy Labs; Mylan; Heritage Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Plendil |
| PubMed Health | Felodipine (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | PLENDIL (felodipine) is a calcium antagonist (calcium channel blocker). Felodipine is a dihydropyridine derivative that is chemically described as ethyl methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate. Its empirica... |
| Active Ingredient | Felodipine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 2.5mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Astrazeneca |
For the treatment of mild to moderate essential hypertension.
FDA Label
Felodipine belongs to the dihydropyridine (DHP) class of calcium channel blockers (CCBs), the most widely used class of CCBs. There are at least five different types of calcium channels in Homo sapiens: L-, N-, P/Q-, R- and T-type. It was widely accepted that CCBs target L-type calcium channels, the major channel in muscle cells that mediates contraction; however, some studies have shown that felodipine also binds to and inhibits T-type calcium channels. T-type calcium channels are most commonly found on neurons, cells with pacemaker activity and on osteocytes. The pharmacologic significance of T-type calcium channel blockade is unknown. Felodipine also binds to calmodulin and inhibits calmodulin-dependent calcium release from the sarcoplasmic reticulum. The effect of this interaction appears to be minor. Another study demonstrated that felodipine attenuates the activity of calmodulin-dependent cyclic nucleotide phosphodiesterase (CaMPDE) by binding to the PDE-1B1 and PDE-1A2 enzyme subunits. CaMPDE is one of the key enzymes involved in cyclic nucleotides and calcium second messenger systems. Felodipine also acts as an antagonist to the mineralcorticoid receptor by competing with aldosterone for binding and blocking aldosterone-induced coactivator recruitment of the mineralcorticoid receptor. Felodipine is able to bind to skeletal and cardiac muscle isoforms of troponin C, one of the key regulatory proteins in muscle contraction. Though felodipine exhibits binding to many endogenous molecules, its vasodilatory effects are still thought to be brought about primarily through inhibition of voltage-gated L-type calcium channels. Similar to other DHP CCBs, felodipine binds directly to inactive calcium channels stabilizing their inactive conformation. Since arterial smooth muscle depolarizations are longer in duration than cardiac muscle depolarizations, inactive channels are more prevalent in smooth muscle cells. Alternative splicing of the alpha-1 subunit of the channel gives felodipine additional arterial selectivity. At therapeutic sub-toxic concentrations, felodipine has little effect on cardiac myocytes and conduction cells.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
C08CA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C08 - Calcium channel blockers
C08C - Selective calcium channel blockers with mainly vascular effects
C08CA - Dihydropyridine derivatives
C08CA02 - Felodipine
Absorption
Is completely absorbed from the gastrointestinal tract; however, extensive first-pass metabolism through the portal circulation results in a low systemic availability of 15%. Bioavailability is unaffected by food.
Route of Elimination
Although higher concentrations of the metabolites are present in the plasma due to decreased urinary excretion, these are inactive. Animal studies have demonstrated that felodipine crosses the blood-brain barrier and the placenta.
Volume of Distribution
10 L/kg
Clearance
0.8 L/min [Young healthy subjects]
Hepatic metabolism primarily via cytochrome P450 3A4. Six metabolites with no appreciable vasodilatory effects have been identified.
17.5-31.5 hours in hypertensive patients; 19.1-35.9 hours in elderly hypertensive patients; 8.5-19.7 in healthy volunteers.
Felodipine decreases arterial smooth muscle contractility and subsequent vasoconstriction by inhibiting the influx of calcium ions through voltage-gated L-type calcium channels. It reversibly competes against nitrendipine and other DHP CCBs for DHP binding sites in vascular smooth muscle and cultured rabbit atrial cells. Calcium ions entering the cell through these channels bind to calmodulin. Calcium-bound calmodulin then binds to and activates myosin light chain kinase (MLCK). Activated MLCK catalyzes the phosphorylation of the regulatory light chain subunit of myosin, a key step in muscle contraction. Signal amplification is achieved by calcium-induced calcium release from the sarcoplasmic reticulum through ryanodine receptors. Inhibition of the initial influx of calcium decreases the contractile activity of arterial smooth muscle cells and results in vasodilation. The vasodilatory effects of felodipine result in an overall decrease in blood pressure. Felodipine may be used to treat mild to moderate essential hypertension.

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?