
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Aktiferrin
2. Apo-ferrous Sulfate
3. Biofer
4. Ceferro
5. Conferon
6. Eisendragees-ratiopharm
7. Eisensulfat Stada
8. Feospan
9. Fer-gen-sol
10. Fer-in-sol
11. Feratab
12. Fero-gradumet
13. Ferodan
14. Ferogradumet
15. Ferro-gradumet
16. Ferrogamma
17. Ferrograd
18. Ferroinfant
19. Ferrous Sulfate, Heptahydrate
20. Ferrous Sulphate
21. Hmatopan
22. Haemoprotect
23. Hemobion
24. Hemofer
25. Iron(ii) Sulfate Heptahydrate
26. Kendural
27. Mol-iron
28. Plastufer
29. Slow-fe
30. Vitaferro Kapseln
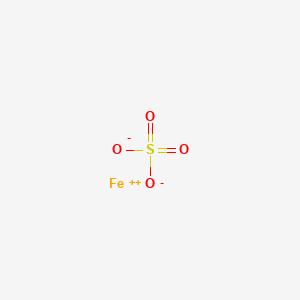
1. Ferrous Salt
2. Iron(ii) Sulfate
3. 7720-78-7
4. Iron Sulfate
5. Iron(2+) Sulfate
6. Iron Sulphate
7. Ferrous Sulfate Anhydrous
8. Iron Sulfate (1:1)
9. Iron(2+) Sulphate
10. Feso4
11. Iron(ii) Sulfate (1:1)
12. Iron Sulfate (feso4)
13. Ferrous Sulfate (1:1)
14. Sulfuric Acid, Iron(2+) Salt (1:1)
15. Sulfuric Acid, Iron(2+) Salt
16. 2idp3x9oud
17. 16547-58-3
18. Iron(2+) Sulfate (anhydrous)
19. Iron Vitriol; Iron(2+) Sulfate
20. Combiron
21. Odophos
22. Kesuka
23. Sal Chalybis
24. Green Salts
25. Quickfloc (salt)
26. Slow Fe
27. Ferrosulfat [german]
28. Ferrosulfat
29. Ccris 6796
30. Hsdb 465
31. Sfe 171
32. Ferrous Sulfate Solution
33. Einecs 231-753-5
34. Unii-2idp3x9oud
35. Nsc 57631
36. Iron(ii) Sulfate Solution
37. Nsc 146177
38. Ai3-51903
39. Ferrous Sulphate Anhydrous
40. Iron(ii)sulphate
41. Fe(ii) Sulphate
42. Iron(ii) Sulphate
43. Einecs 240-616-9
44. Iron(2+);sulfate
45. Iron (as Sulphate)
46. Iron (ii) Sulphate
47. Ferrous Sulfate,dried
48. Errous Hydrogen Sulfide
49. Sulfuric Acid, Iron(2+) Salt (1:?)
50. Iron Sulphate (feso4)
51. Ferrous Sulfate, 98%
52. Ferrous Sulfate (anh.)
53. Fe(ii)so4
54. Dsstox_cid_9688
55. Ferrous Sulfate, Anhydrous
56. Ferrous Sulphate (1:1)
57. Ec 231-753-5
58. Ferrous Sulphate, Anhydrous
59. Ferrous Sulfate (anhydrous)
60. Dsstox_rid_78808
61. Iron(2+) Sulfate (anh.)
62. Dsstox_gsid_29688
63. Ferrous Sulfate [mi]
64. Iron(ii) Sulfate (feso4)
65. Ferrous Sulfate [iso]
66. Ferrous Sulfate [hsdb]
67. Dtxsid0029688
68. Chebi:75832
69. Ferrous Sulfate [who-dd]
70. Ferrous Sulphate Exsiccated (dried)
71. Iron(ii) Sulphate (1:1)
72. Tox21_202580
73. Ferrous Sulfate,dried [vandf]
74. Db13257
75. Ncgc00260129-01
76. Cas-7720-78-7
77. Ft-0626420
78. Q214863
79. 8063-79-4
| Molecular Weight | 151.91 g/mol |
|---|---|
| Molecular Formula | FeO4S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 151.886665 g/mol |
| Monoisotopic Mass | 151.886665 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Initial response to iron therapy ...will confirm or negate diagnosis of iron-deficiency anemia. Ferrous sulfate, 150-300 mg thrice daily, is given for 3 wk. Beginning about a wk after starting therapy, circulating hemoglobin should rise about 0.1-0.3 g % daily; less severe anemia, less daily rise.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1290
Supplementation with 30-60 mg of iron daily (ie, 150-300 mg of ferrous sulfate) has been advocated for pregnant women ...and 0.3-0.6 mL of ferrous sulfate pediatric "drops" daily for low-birth-weight infants from 1 month until 1 yr of age. Physician must use his judgement in this regard... .
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1290
Hematinic.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 717
MEDICATION (VET): In iron deficiency. Astringent.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 717
For more Therapeutic Uses (Complete) data for FERROUS SULFATE (11 total), please visit the HSDB record page.
... A double-lumen perfusion tube was positioned orogastrically into a 40-cm segment of the proximal small intestine in six healthy volunteers (25 +/- 5 yr). The segment was perfused with saline and subsequently with saline containing 80 mg iron as ferrous sulfate at a rate of 10 mL/min. Intestinal fluid samples were collected at 15-min intervals. Thiobarbituric acid reactive substances concentrations as an indicator of lipid peroxidation increased significantly from 0.07 uM (range, 0-0.33 uM) during saline perfusion to 3.35 uM (range, 1.19-7.27 uM) during iron perfusion (P<0.05). Nonprotein antioxidant capacity increased significantly from 474 uM (range, 162-748 uM) to 1,314 uM (range, 674-1,542 uM) (P<0.05). These data show that a single dosage of ferrous sulfate induces oxidative damage and the subsequent release of an antioxidant in the small intestine in vivo in healthy volunteers.
Troost FJ et al; Am J Physiol Gastrointest Liver Physiol 285 (2): G354-9 (2003)
Adverse effects of ferrous sulfate are those of iron compounds in general, but they are rarely severe when drug is taken in therapeutic doses; however, relatively small overdoses can cause serious intoxication in infants and children.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 780
VET: Administer with or after feeding to help avoid gastritis.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 221
Adverse effects are generally dose dependent; in 10% of patients they are severe enough to be intolerable. Gastrointestinal disturbances ... are most common. Ferrous sulfate is absorbed best when taken between meals, but gastrointestinal symptoms may be minimized by reducing the dose and/or giving it in divided amounts with meals or shortly thereafter. In some patients, admin of one-half the total daily dose at bedtime improves tolerance. ... Large doses of ferrous sulfate may aggravate existing gastrointestinal diseases ... . Acute severe iron poisoning is uncommon in adults but does occur in children who ingest formulations intended for adults. In young children, as little as 400 mg of elemental iron is potentially fatal.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 797
For more Drug Warnings (Complete) data for FERROUS SULFATE (9 total), please visit the HSDB record page.
Ferrous sulfate is used for the prevention and treatment of iron deficiency anemia in adults and children.
Ferrous sulfate replenishes iron, an essential component in hemoglobin, myoglobin, and various enzymes. It replaces the iron that is usually found in hemoglobin and myoglobin. Iron participates in oxygen transport and storage, electron transport and energy metabolism, antioxidant and beneficial pro-oxidant functions, oxygen sensing, tissue proliferation and growth, as well as DNA replication and repair.
B - Blood and blood forming organs
B03 - Antianemic preparations
B03A - Iron preparations
B03AA - Iron bivalent, oral preparations
B03AA07 - Ferrous sulfate
B - Blood and blood forming organs
B03 - Antianemic preparations
B03A - Iron preparations
B03AD - Iron in combination with folic acid
B03AD03 - Ferrous sulfate
Absorption
Approximately 5 10% of dietary iron is absorbed, and this absorption rate increases to up to 30% in iron deficiency states. Oral iron supplements are absorbed up to 60% via active and passive transport processes. Gastrointestinal absorption of iron occurs via strict regulation by the enterocyte and duodenal cytochrome and ferric reductase enzymes. The hormone hepcidin heavily regulates iron absorption and distribution throughout the body. The median time to maximum serum concentration (Tmax) is generally 4 hours after administration. Between 2-8 hours post administration, average serum iron concentrations fluctuate by 20%, according to one study. Bioavailability of iron depends on whether it is administered in a film coated tablet or enteric coated tablet. One pharmacokinetic study in healthy volunteers revealed a 30% bioavailability for enteric coated tablets. The AUC of enteric coated tablets varied between a lower limit of -46.93 to 5.25 molxh/l. Cmax is higher for film coated tablets, ranging from 3.4 to 22.1 mol/h/l. It is advisable to take ferrous sulfate with ascorbic acid, as this practice may increase absorption. Avoid antacids, tea, coffee,tea, dairy products, eggs, and whole-grain bread for at least an hour after taking ferrous sulfate. Calcium can decrease iron absorption by 33% if taken concomitantly.
Route of Elimination
Oral iron is recycled, with some loss in the urine, sweat, and desquamation. Some iron can be lost during menstrual bleeding This loss is balanced by changes in intestinal absorption. The enzyme hepcidin promotes the excretion of iron via the sloughing of enterocytes with ferritin stores into the feces.
Volume of Distribution
About 60% of iron is distributed the erythrocytes. The remainder of the iron is found in muscle tissues (as a part of myoglobin), and in a variety of different enzymes, as well as in storage form. Most stored iron is in the form of ferritin, which can be found in the liver, bone marrow, spleen and, and muscle. Iron crosses the placenta and is also found in breast milk.
... The bioavailability studies were carried out using four groups of 30 female mice each. In two groups, we studied the absorption of ferrous ascorbate and ferrous sulfate, both in water as reference standards, which show absorptions of 13.1+/-4.9% and 13.2+/-4.3%, respectively. With the third group, we studied the absorption of ferrous sulfate in milk; its value, 7.9+/-3.2%, is significantly lower than that of the remaining groups, with a p < 0.01. The studies with SFE-171 in milk, were performed on the fourth group, with a result of 11.6+/-4.5%, demonstrating that its absorption does not differ significantly from that of the reference standards. The absorption mechanism was determined by means of in vivo self-displacement studies of the ferrous ion and the SFE-171, taking ferrous sulfate as the reference compound. For this study, 210 female mice were used, and no significant difference between the absorption mechanism of both products could be observed.
PMID:9630425 Boccio JR et al; Biol Trace Elem Res 62 (1-2): 65-73 (1998)
We investigated the iron bioavailability of microencapsulated ferrous sulfate (SFE-171) in a diet based on powdered milk by using the prophylactic method in rats.The SFE-171 was added into fluid milk and industrially processed into powdered milk, which was then mixed in our laboratory with a normalized diet (17.2 +/- 2.1 mg Fe/kg). A reference standard diet using ferrous sulfate as iron-fortifying source (19.8 p+/- 2.9 mg Fe/kg) and a control diet without added iron (4.6 +/- 0.8 mg Fe/kg) were prepared in the laboratory in a similar way. These diets were administered to different groups of weaning rats for 28 d as the only solid nourishment. The iron bioavailability of the different sources was calculated as the relation between the mass of iron incorporated into hemoglobin during the treatment and the total iron intake per animal. The iron bioavailability values of SFE-171 and ferrous sulfate in the fortified diets were 41.6 +/- 6.6% and 42.6 +/- 4.2%, respectively; these results were significantly higher (P < 0.01) than the iron bioavailability of the control diet (28.8 +/- 8.1%).
Lysionek AE et al; Nutr 18 (3): 279-281 (2002)
A prospective analytical study was conducted to determine the relationship between nonprotein-bound iron and serum iron concentrations following gastric instillation of ferrous sulfate. Four female pigs (2022 kg) with indwelling central venous lines and gastrostomy tubes were studied. A 5% solution of ferrous sulfate (20 mg elemental iron/kg bwt) was administered through the gastrostomy tube over 1 to 2 min. Six hourly blood samples were collected, and serum samples were subjected to ultrafiltration with the filtrate representing nonprotein-bound iron. Iron concentrations were determined by atomic absorption spectrophotometry. Baseline (mean | SD) iron concentrations were 73 | 25 mug/dL as the serum total and 21 | 4 mug/dL as nonprotein-bound iron. The serum iron and nonprotein-bound iron concentrations achieved a peak of 191 | 66 and 23 | 10, respectively, at 2 h and declined to near baseline values at 6 h. The mean ratio of filtrate to serum iron concentration was 0.
PMID:8825744 Chyka PA et al; Vet Hum Toxicol 38 (1): 24-26 (1996)
Gastrointestinal absorption of iron is adequate and essentially equal from...ferrous...sulfate, fumarate, gluconate, succinate, glutamate, and lactate.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1315
The metabolism of iron is complex. Normally, iron exists in the ferrous (Fe2+) or ferric (Fe3+) state, but since Fe2+ is oxidized to Fe3+, which hydrolyzes to insoluble iron(III)hydroxides in neutral aqueous solutions, iron binds to plasma proteins and is either transported or stored throughout the body. There are three proteins that serve to regulate the storage and transport of ingested iron. The first protein , transferrin, transports iron in both the plasma and extracellular fluid. Ceruloplasmin in the plasma and hephaestin on the enterocyte participate in the oxidation and binding of iron to transferrin. The main role of transferrin is the chelation of iron to prevent the production of reactive oxygen species, while facilitating its transport into cells. The transferrin receptor, located on many cells that require iron, binds the transferrin complex and internalizes this complex. Ferritin is a protein that stores iron, making it readily available for body requirements.
The half-life of orally administered iron is not readily available in the literature, with total effects lasting 2-4 months (congruent with the red blood cell life span) with an onset of action of 4 days and peak activity at 7-10 days.
Iron is required to maintain optimal health, particularly for helping to form red blood cells (RBC) that carry oxygen around the body. A deficiency in iron indicates that the body cannot produce enough normal red blood cells. Iron deficiency anemia occurs when body stores of iron decrease to very low levels, and the stored iron is insufficient to support normal red blood cell (RBC) production. Insufficient dietary iron, impaired iron absorption, bleeding, pregnancy, or loss of iron through the urine can lead to iron deficiency. Symptoms of iron deficiency anemia include fatigue, breathlessness, palpitations, dizziness, and headache. Taking iron in supplement form, such as ferrous sulfate, allows for more rapid increases in iron levels when dietary supply and stores are not sufficient. Iron is transported by the divalent metal transporter 1 (DMT1) across the endolysosomal membrane to enter the macrophage. It can then can be incorporated into ferritin and be stored in the macrophage or carried of the macrophage by ferroportin. This exported iron is oxidized by the enzyme to ceruloplasmin to Fe3+, followed by sequestration by transferrin for transport in the serum to various sites, including the bone marrow for hemoglobin synthesis or into the liver. Iron combines with porphyrin and globin chains to form hemoglobin, which is critical for oxygen delivery from the lungs to other tissues.
Coagulopathy is a hallmark of severe ferrous sulfate poisoning in humans and lab animals. At iron concn comparable to those of previous animal investigations, the coagulopathy, in other words, the dose-related prolongation of the prothrombin, thrombin, and partial thromboplastin time, was reproduced in human plasma in vitro. Studies of the mechanism by which iron prevents a normal plasma coagulation revealed that the proenzymes of the coagulation cascade and fibrinogen were not damaged by iron. Fibrinogen coagulability and fibrin monomer aggregation were unaffected by very high iron concn. Instead, thrombin was markedly inhibited by iron in its clotting effect on fibrinogen and, specifically, in its fibrinopeptide A-generating capacity, the inhibitory effect being reversible upon iron removal by ethylenediaminetetraacetic acid chelation and gel filtration. Thrombin generation in the presence of iron was reduced as well, indicating an inhibition of one or several other enzymes of the intrinsic coagulation cascade. Because the amidolytic activity of human thrombin as well as factor Xa, kallikrein, and bovine trypsin was also reversibly suppressed by ferrous sulfate, it is considered likely that coagulopathy occurring in iron poisoning is the consequence of a general, physiologically important phenomenon: the susceptibility of serine proteases to nontransferrin-bound iron(3+)
PMID:6421970 Rosenmund A et al; J Lab Clin Med 103 (4): 524-33 (1984)
The mechanism of acute iron cardiotoxicity was investigated in isometrically contracting left atrial strips and right ventricular papillary muscles isolated from rabbit hearts. A 90 min exposure to iron (1.8 mM; as ferrous sulfate) reduced the peak-developed tension and the maximal rate of tension development.
PMID:6695379 Artman M et al; Toxicol Appl Pharmacol 72 (2): 324-32 (1984)
... Iron is stored in the liver in the oxidized or ferric state & is tightly bound to protein as ferric ferritin. Xanthine oxidase appears to be involved in the conversion of ferric ferritin to ferrous ferritin. The reduced form of iron is less tightly bound to ferritin and thus is more easily released for utilization. Therefore, a possible inverse relationship between hepatic xanthine oxidase activity & hepatic iron storage exists. Theoretically, the xanthine oxidase inhibitor allopurinol should decrease the activity of xanthine oxidase & increase hepatic iron storage.
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 16/7
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?