
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
FDF
0
Australia

0
South Africa

0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
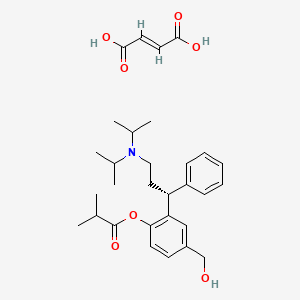

1. Fesoterodine
2. Toviaz
1. 286930-03-8
2. Toviaz
3. (r)-fesoterodine Fumarate
4. Spm 907
5. Spm 8272
6. Spm-907
7. Spm-8272
8. Fesoterodine Maleate
9. Fesoterodine (fumarate)
10. Eos72165s7
11. (e)-but-2-enedioic Acid;[2-[(1r)-3-[di(propan-2-yl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl] 2-methylpropanoate
12. Propanoic Acid, 2-methyl-, 2-[(1r)-3-[bis(1-methylethyl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl Ester, (2e)-2-butenedioate (1:1)
13. 286930-03-8 (fumarate); 286930-02-7 (free Base)
14. Fesoterodine Fumarate [usan]
15. Unii-eos72165s7
16. Fesoterodine Fumarate [usan:jan]
17. (e)-but-2-enedioic Acid,[2-[(1r)-3-[di(propan-2-yl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl] 2-methylpropanoate
18. Toviaz (tn)
19. (r)-fesoterodinefumarate
20. Mls003915638
21. Schembl814971
22. Fesoterodine Fumarate - Toviaz
23. Schembl1993632
24. Chembl1201765
25. Dtxsid00904655
26. Bcpp000231
27. Fesoterodine Fumarate (jan/usan)
28. Fesoterodine Fumarate [mi]
29. Hms3884d11
30. Fesoterodine Fumarate [jan]
31. Amy37611
32. Hy-a0030
33. Bdbm50248002
34. Fesoterodine Fumarate [vandf]
35. Mfcd12756004
36. S2240
37. Fesoterodine Fumarate [mart.]
38. Akos005146248
39. Akos015855886
40. Fesoterodine Fumarate [who-dd]
41. Ac-3486
42. Bcp9000682
43. Ccg-269901
44. Cs-0822
45. Ks-1298
46. 2-((1r)-3-(diisopropylamino)-1-phenylpropyl)-4-(hydroxymethyl)phenyl Isobutyrate
47. Fesoterodine Fumarate [ema Epar]
48. 2-((1r)-3-(bis(1-methylethyl)amino)-1-phenylpropyl)-4-(hydroxymethyl)phenyl 2-methylpropanoate Hydrogen (2e)-butenedioate (salt)
49. Propanoic Acid, 2-methyl-, 2-((1r)-3-(bis(1-methylethyl)amino)-1-phenylpropyl)-4-(hydroxymethyl)phenyl Ester, (2e)-2-butenedioate (1:1) (salt)
50. Smr002544691
51. Fesoterodine Fumarate [orange Book]
52. Pf-00695838
53. D08923
54. 930f038
55. A846296
56. Q27277274
57. 2-((1r)-3-(bis(1-methylethyl)amino)-1-phenylpropyl)-4-(hydroxymethyl)phenyl 2-methylpropanoate Hydrogen(2e)-butenedioate (salt)
58. Fesoterodinefumarate;(r)-fesoterodine Fumarate;2-methylpropanoic Acid 2-[(1r)-3-[bis(1-methylethyl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl Ester (2e)-2-butenedioate
59. Propanoic Acid,2-methyl ,2-((1r)-3-(bis(1-methylethyl)amino)-1-phenylpropyl)-4-(hydroxymethyl)phenyl Ester,(2e)-2-butenedioate (1:1)(salt)
| Molecular Weight | 527.6 g/mol |
|---|---|
| Molecular Formula | C30H41NO7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 13 |
| Exact Mass | 527.28830265 g/mol |
| Monoisotopic Mass | 527.28830265 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 610 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Toviaz |
| PubMed Health | Fesoterodine (By mouth) |
| Drug Classes | Urinary Antispasmodic |
| Drug Label | Toviaz contains fesoterodine fumarate and is an extended-release tablet. Fesoterodine is rapidly de-esterified to its active metabolite (R)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethyl-phenol, or 5-hydroxymethyl tolterodine, which is a musca... |
| Active Ingredient | Fesoterodine fumarate |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 8mg; 4mg |
| Market Status | Prescription |
| Company | Pfizer |
| 2 of 2 | |
|---|---|
| Drug Name | Toviaz |
| PubMed Health | Fesoterodine (By mouth) |
| Drug Classes | Urinary Antispasmodic |
| Drug Label | Toviaz contains fesoterodine fumarate and is an extended-release tablet. Fesoterodine is rapidly de-esterified to its active metabolite (R)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethyl-phenol, or 5-hydroxymethyl tolterodine, which is a musca... |
| Active Ingredient | Fesoterodine fumarate |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 8mg; 4mg |
| Market Status | Prescription |
| Company | Pfizer |
Treatment of the symptoms (increased urinary frequency and / or urgency and / or urgency incontinence) that may occur in patients with overactive-bladder syndrome.
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Urological Agents
Drugs used in the treatment of urological conditions and diseases such as URINARY INCONTINENCE and URINARY TRACT INFECTIONS. (See all compounds classified as Urological Agents.)
G04BD11

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?