
Synopsis
Synopsis
0
VMF
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
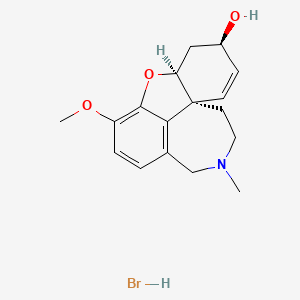

1. Galantamin
2. Galantamine
3. Galanthamine
4. Galanthamine Hydrobromide
5. Lycoremine
6. Nivalin
7. Nivaline
8. Razadyne
9. Reminyl
1. Galanthamine Hydrobromide
2. 1953-04-4
3. Reminyl
4. Nivalin
5. Nivaline
6. Razadyne
7. Lycoremine Hydrobromide
8. Jilkon Hydrobromide
9. Galanthamine (hydrobromide)
10. Galanthamine Hbr
11. Tamilin
12. 193146-85-9
13. Mj4ptd2vvw
14. 5n4sa4kqx9
15. (-)-galantamine Hydrobromide
16. (+/-)-galantamine Hydrobromide
17. (+/-)-galanthamine Hydrobromide
18. Galantamine Hydrobromide (racemic)
19. Galantamine Hydrobromide, (+/-)-
20. (1s,12s,14r)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15-tetraen-14-ol;hydrobromide
21. (4as,6r,8as)-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6h-benzofuro[3a,3,2-ef][2]benzazepin-6-ol Hydrobromide
22. Razadyne Er
23. Reminyl (tn)
24. (1s,12s,14r)-9-methoxy-4-methyl-11-oxa-4-azoniatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15-tetraen-14-ol;bromide
25. (4as,6r,8as)-3-methoxy-11-methyl-4a,5,9,10,11,12-hexahydro-6h-benzo[2,3]benzofuro[4,3-cd]azepin-6-ol Hydrobromide
26. Galanthaminehydrobromide
27. 1953-04-4 (hbr); 1953-04-4 (free Base).
28. (1s,12s,14r)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.0^{1,12}.0^{6,17}]heptadeca-6(17),7,9,15-tetraen-14-ol Hydrobromide
29. (4as,6r,8as)-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol Hydrobromide
30. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, Hydrobromide (1:1), (4ar,6s,8ar)-rel-
31. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, Hydrobromide (1:1), (4as,6r,8as)-
32. Smr000449267
33. Sr-01000597844
34. Nivaline (pharmaceutical)
35. C17h22brno3
36. Anti-alzheimer
37. Sr-05000001783
38. Nivalin;razadyne
39. Galantamine Hydrobromide [usan]
40. Reminyl Xl
41. Jilcon Hydrobromide
42. Razadyne (tn)
43. Prestwick_236
44. Einecs 217-780-5
45. Galanthamine Hydrobromide From Lycoris Sp.
46. Mfcd00067672
47. Unii-mj4ptd2vvw
48. Unii-5n4sa4kqx9
49. 1953-04-4 Unlabeled
50. Chembl1555
51. Galanthamine-d3 Hydrobromide
52. Mls000758283
53. Mls001401401
54. Galanthamine Hydrobromide,(s)
55. Schembl177993
56. Spectrum1501202
57. Hms1569f18
58. Hms1921p21
59. Hy-a0009
60. Galantamine Hydrobromide (jan/usp)
61. Ac-469
62. Ccg-38829
63. Galantamine Hydrobromide [mi]
64. Galantamine Hydrobromide [usan:usp]
65. S1339
66. Galantamine Hydrobromide [jan]
67. Galanthamine-o-methyl-d3 Hydrobromide
68. Akos007930166
69. Akos015960209
70. Cs-0378
71. Fd10095
72. Galantamine Hydrobromide [hsdb]
73. Nc00061
74. Galantamine Hydrobromide [mart.]
75. Galantamine Hydrobromide [vandf]
76. Galantamine Hydrobromide [usp-rs]
77. Galantamine Hydrobromide [who-dd]
78. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, Hydrobromide, (4as,6r,8as)-
79. As-12155
80. Gp-37267
81. G0293
82. Galantamine Hydrobromide [orange Book]
83. D02173
84. Galantamine Hydrobromide [ep Monograph]
85. Galantamine Hydrobromide [usp Monograph]
86. Galantamine Hydrobromide Racemic [usp-rs]
87. 953g044
88. A866857
89. A903748
90. R-113675
91. Sr-01000597844-1
92. Sr-01000597844-5
93. Sr-05000001783-3
94. Q47495772
95. Z1558572528
96. Galanthamine Hydrobromide From Lycoris Sp., >=94% (tlc)
97. (4as,6r,8as)-3-methoxy-11-methyl-5,6,9,10,11,12-hexahydro-4ah-benzo[2,3]benzofuro[4,3-cd]azepin-6-ol Hydrobromide
98. (4as,6r,8as)-3-methoxy-11-methyl-5,6,9,10,11,12-hexahydro-4ah-benzo[2,3]benzofuro[4,3-cd]azepin-6-olhydrobromide
99. (4as,6r,8as)-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6h-benzofuro[3a,3,2-ef][2]benzazepin-6-ol, Hydrobromide
100. (4as,6r,8as)-4a,5,9,10,11,12-hexahydro-3-methoxy-d3-11-methyl-6h-benzofuro[3a,3,2-ef][2]benzazepin-6-ol Hydrobromide
101. 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6h-benzofuro[3a,3,2-ef][2]benzazepin-6-ol Hydrobromide
102. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, Hbr (1:1), (4as,6r,8as)-
103. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, Hbr, (4as,6r,8as)-
104. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, Hydrobromide, (4a.alpha.,6.beta.,8ar*)-
105. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, Hydrobromide, (4aalpha,6beta,8ar*)-
1. Galantamine
2. Galanthamine Hydrobromide
3. Lycoremin
4. Lycoremine
5. Nivalin
6. Razadyne
7. Reminyl
8. Galanthamine
| Molecular Weight | 368.3 g/mol |
|---|---|
| Molecular Formula | C17H22BrNO3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 367.07831 g/mol |
| Monoisotopic Mass | 367.07831 g/mol |
| Topological Polar Surface Area | 41.9 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 440 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Galantamine hydrobromide |
| PubMed Health | Galantamine (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Drug Label | Galantamine hydrobromide is a reversible, competitive acetylcholinesterase inhibitor. Galantamine hydrobromide is known chemically as (4aS,6R,8aS)-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6H-benzofuro [3a,3,2-ef][2]benzazepin-6-ol hydrobromide.... |
| Active Ingredient | Galantamine hydrobromide |
| Dosage Form | Tablet; Capsule, extended release; Solution |
| Route | oral; Oral |
| Strength | eq 4mg base; 8mg; eq 12mg base; 4mg; 4mg/ml; 12mg; eq 16mg base; eq 24mg base; eq 8mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Ranbaxy; Apotex; Aurobindo Pharma; Sun Pharm Inds; Sandoz; Sun Pharma Global; Roxane; Watson Labs; Teva Pharms; Zydus Pharms Usa; Dr Reddys Labs; Mylan; Impax Labs; Barr |
| 2 of 4 | |
|---|---|
| Drug Name | Razadyne |
| Drug Label | RAZADYNE ER/RAZADYNE (galantamine hydrobromide) is galantamine hydrobromide, a reversible, competitive acetylcholinesterase inhibitor. Galantamine hydrobromide is known chemically as (4aS ,6R,8aS )-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6H... |
| Active Ingredient | Galantamine hydrobromide |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | eq 4mg base; eq 12mg base; 4mg/ml; eq 8mg base |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Galantamine hydrobromide |
| PubMed Health | Galantamine (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Drug Label | Galantamine hydrobromide is a reversible, competitive acetylcholinesterase inhibitor. Galantamine hydrobromide is known chemically as (4aS,6R,8aS)-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6H-benzofuro [3a,3,2-ef][2]benzazepin-6-ol hydrobromide.... |
| Active Ingredient | Galantamine hydrobromide |
| Dosage Form | Tablet; Capsule, extended release; Solution |
| Route | oral; Oral |
| Strength | eq 4mg base; 8mg; eq 12mg base; 4mg; 4mg/ml; 12mg; eq 16mg base; eq 24mg base; eq 8mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Ranbaxy; Apotex; Aurobindo Pharma; Sun Pharm Inds; Sandoz; Sun Pharma Global; Roxane; Watson Labs; Teva Pharms; Zydus Pharms Usa; Dr Reddys Labs; Mylan; Impax Labs; Barr |
| 4 of 4 | |
|---|---|
| Drug Name | Razadyne |
| Drug Label | RAZADYNE ER/RAZADYNE (galantamine hydrobromide) is galantamine hydrobromide, a reversible, competitive acetylcholinesterase inhibitor. Galantamine hydrobromide is known chemically as (4aS ,6R,8aS )-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6H... |
| Active Ingredient | Galantamine hydrobromide |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | eq 4mg base; eq 12mg base; 4mg/ml; eq 8mg base |
| Market Status | Prescription |
| Company | Janssen Pharms |
Nootropic Agents
Drugs used to specifically facilitate learning or memory, particularly to prevent the cognitive deficits associated with dementias. These drugs act by a variety of mechanisms. (See all compounds classified as Nootropic Agents.)
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
Parasympathomimetics
Drugs that mimic the effects of parasympathetic nervous system activity. Included here are drugs that directly stimulate muscarinic receptors and drugs that potentiate cholinergic activity, usually by slowing the breakdown of acetylcholine (CHOLINESTERASE INHIBITORS). Drugs that stimulate both sympathetic and parasympathetic postganglionic neurons (GANGLIONIC STIMULANTS) are not included here. (See all compounds classified as Parasympathomimetics.)
 Capital Farma, a leading European pharmaceutical company focusing on the development & distribution of niche APIs & Pharma Services.
Capital Farma, a leading European pharmaceutical company focusing on the development & distribution of niche APIs & Pharma Services.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.

GDUFA
DMF Review : Reviewed
Rev. Date : 2014-12-05
Pay. Date : 2012-11-08
DMF Number : 18112
Submission : 2005-02-23
Status : Active
Type : II

Certificate Number : R1-CEP 2011-053 - Rev 02
Issue Date : 2020-02-20
Type : Chemical
Substance Number : 2366
Status : Valid


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17972
Submission : 2005-01-05
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17967
Submission : 2005-01-03
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 14425
Submission : 1999-09-29
Status : Inactive
Type : II

Certificate Number : R1-CEP 2012-248 - Rev 01
Issue Date : 2021-11-19
Type : Chemical
Substance Number : 2366
Status : Valid

NDC Package Code : 12578-890
Start Marketing Date : 2001-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Korea Janssen Co., Ltd.
Registration Date : 2021-06-10
Registration Number : 20210610-209-J-538
Manufacturer Name : Janssen Pharmaceutica NV
Manufacturer Address : Jassen Pharmaceuticalaan 3, Geel, 2440, Belgium

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15384
Submission : 2001-05-08
Status : Inactive
Type : II

NDC Package Code : 12578-890
Start Marketing Date : 2001-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Korea Janssen Co., Ltd.
Registration Date : 2021-06-10
Registration Number : 20210610-209-J-538
Manufacturer Name : Janssen Pharmaceutica NV
Manufacturer Address : Jassen Pharmaceuticalaan 3, Geel, 2440, Belgium

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18152
Submission : 2005-03-03
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18045
Submission : 2005-01-28
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15384
Submission : 2001-05-08
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 14425
Submission : 1999-09-29
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18114
Submission : 2005-02-22
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2015-08-24
Pay. Date : 2015-08-17
DMF Number : 17863
Submission : 2004-12-01
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-12-05
Pay. Date : 2012-11-08
DMF Number : 18112
Submission : 2005-02-23
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17967
Submission : 2005-01-03
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17972
Submission : 2005-01-05
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Galantamine hydrobromide, Produced by a synthetic process
Certificate Number : CEP 2009-316 - Rev 02
Status : Valid
Issue Date : 2024-01-16
Type : Chemical
Substance Number : 2366

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Galantamine hydrobromide, Isolated from natural sources
Certificate Number : R1-CEP 2011-288 - Rev 01
Status : Valid
Issue Date : 2020-11-19
Type : Chemical
Substance Number : 2366

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Galantamine hydrobromide, Produced by a synthetic process
Certificate Number : R1-CEP 2012-248 - Rev 01
Status : Valid
Issue Date : 2021-11-19
Type : Chemical
Substance Number : 2366

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Galantamine hydrobromide, Produced by a synthetic process
Certificate Number : R1-CEP 2011-123 - Rev 02
Status : Valid
Issue Date : 2022-08-12
Type : Chemical
Substance Number : 2366

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Galantamine hydrobromide, produced by a synthetic process
Certificate Number : CEP 2023-389 - Rev 00
Status : Valid
Issue Date : 2024-06-19
Type : Chemical
Substance Number : 2366

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Galantamine hydrobromide, Produced by a synthetic process
Certificate Number : R1-CEP 2012-328 - Rev 00
Status : Withdrawn by Holder
Issue Date : 2019-03-07
Type : Chemical
Substance Number : 2366

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Galantamine hydrobromide, Produced by a synthetic process
Certificate Number : CEP 2011-011 - Rev 02
Status : Valid
Issue Date : 2024-10-21
Type : Chemical
Substance Number : 2366

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Galantamine hydrobromide, Isolated from natural sources
Certificate Number : R1-CEP 2011-053 - Rev 02
Status : Valid
Issue Date : 2020-02-20
Type : Chemical
Substance Number : 2366

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]  DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
NDC Package Code : 55111-081
Start Marketing Date : 2005-01-20
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
NDC Package Code : 65862-487
Start Marketing Date : 2024-01-11
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 12578-890
Start Marketing Date : 2001-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 65372-1138
Start Marketing Date : 2008-11-28
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 65129-1332
Start Marketing Date : 2013-09-02
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 65129-1113
Start Marketing Date : 2004-04-29
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 47848-023
Start Marketing Date : 2022-01-26
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (100kg/100kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : GALANTAMINE HYDROBROMIDE
Dosage Form : TABLET; ORAL
Dosage Strength : EQ 4MG BASE
Approval Date :
Application Number : 77767
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : GALANTAMINE HYDROBROMIDE
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 8MG BASE
Approval Date : 2011-03-29
Application Number : 90957
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : GALANTAMINE HYDROBROMIDE
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 12MG BASE
Approval Date : 2009-09-15
Application Number : 77585
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : GALANTAMINE HYDROBROMIDE
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 4MG BASE
Approval Date : 2009-07-09
Application Number : 77587
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : GALANTAMINE HYDROBROMIDE
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 12MG BASE
Approval Date : 2009-02-11
Application Number : 77608
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : GALANTAMINE HYDROBROMIDE
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : EQ 16MG BASE
Approval Date : 2009-05-27
Application Number : 78484
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : GALANTAMINE HYDROBROMIDE
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : EQ 24MG BASE
Approval Date : 2009-05-27
Application Number : 78484
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : GALANTAMINE HYDROBROMIDE
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 8MG BASE
Approval Date : 2009-05-29
Application Number : 77590
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

Brand Name : GALANTAMINE HYDROBROMIDE
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 4MG BASE
Approval Date : 2008-08-28
Application Number : 77603
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Brand Name : GALANTAMINE HYDROBROMIDE
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 8MG BASE
Approval Date : 2008-08-28
Application Number : 77603
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Application : Coating Systems & Additives
Excipient Details : ACTILLETS™ are microcrystalline cellulose spheres used in advanced drug formulations as starter cores for drug layering and coating.
Pharmacopoeia Ref : NA
Technical Specs : Bulk density: 0.80
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Orodispersible Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Orodispersible Tablet
Grade : Oral
Dosage Form : Orodispersible Tablet
Grade : Oral
Application : Chewable & Orodispersible Aids
Excipient Details : CS90 is a directly compressible calcium carbonate with starch used for chewable tablets due to its smooth mouthfeel and creamy texture.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 150-175, Tapped Density: 0.85
Ingredient(s) : Calcium Carbonate Excipient
Dosage Form : Orodispersible Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Orodispersible Tablet
Grade : Oral
Application : Chewable & Orodispersible Aids
Excipient Details : MS90 is a directly compressible magnesium hydroxide with starch used for chewable tablets due to its smooth mouthfeel and creamy texture.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 150-170, Taped Density: 0.80
Ingredient(s) : Starch
Dosage Form : Orodispersible Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Injectable / Parenteral
Grade : Parenteral
Brand Name : Expansorb® PLA / PEG
Application : Parenteral
Excipient Details : Expansorb® PLA?/PEG? polymers are used as functional excipients in injectable formulations for controlled & slow drug release with a single injection.
Pharmacopoeia Ref : N/A
Technical Specs : Classic and ultrapure LMP
Ingredient(s) : Poly lactic acid
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Brand Name : Expansorb® PLGA / PEG
Application : Parenteral
Excipient Details : Expansorb® PLGA? / PEG copolymers are used as functional excipients in single dose injections for controlled and slow drug release (weeks to months).
Pharmacopoeia Ref : N/A
Technical Specs : Classic and ultrapure LMP
Ingredient(s) : Poly-DL-Lactic-co-Glycolic Acid
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Injectable / Parenteral
Grade : Parenteral, Oral
Dosage Form : Injectable / Parenteral
Grade : Parenteral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?