
Synopsis
0
EU WC
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
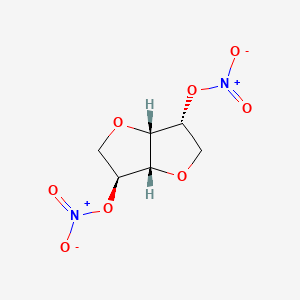

1. Cardonit 40
2. Dilatrate
3. Dinitrate, Isosorbide
4. Iso Bid
5. Iso-bid
6. Isobid
7. Isodinit
8. Isoket
9. Isoket Retard 120
10. Isoket Retard-120
11. Isoket Retard120
12. Isomak R
13. Isordil
14. Isotrate
15. Nitrosorbide
16. Sorbitrate
17. Sorbonit
1. 87-33-2
2. Isordil
3. Sorbide Nitrate
4. Sorbidnitrate
5. Nitrosorbide
6. Sorbitrate
7. Dinitrosorbide
8. Isoket
9. Dilatrate-sr
10. Sorbidilat
11. Vascardin
12. Carvasin
13. Flindix
14. Isorbid
15. Isotrate
16. Sorbonit
17. Cardis
18. Isdn
19. Carvanil
20. Cedocard
21. Claodical
22. Cornilat
23. Corosorbide
24. Difutrat
25. Dilatrate
26. Harrical
27. Iso-bid
28. Myorexon
29. Nitrosorbid
30. Nitrosorbon
31. Resoidan
32. Sorbangil
33. Sorbislo
34. Vasodilat
35. Vasorbate
36. Coronex
37. Emoper
38. Korodil
39. Lomilan
40. Maycor
41. Rigedal
42. Sorquad
43. Tinidil
44. Nosim
45. Xanyl
46. Sorbide, Dinitrate
47. Isordil Tembids
48. Rifloc Retard
49. Dinitroisosorbide
50. Cardio 10
51. Dianhydrosorbitol 2,5-dinitrate
52. D-isosorbide Dinitrate
53. Isosorbide 2,5-dinitrate
54. Isodinit
55. Isostat
56. Ibd 20
57. Isosorbidi Dinitras
58. Sorbide T.d.
59. Dinitrate D'isosorbide
60. Dinitrato De Isosorbida
61. Astridine
62. Isochron
63. D-glucitol, 1,4:3,6-dianhydro-, Dinitrate
64. Sorbidinitrate
65. Corovliss
66. Disorlon
67. Langoran
68. Sorbide
69. Sorquat
70. 1,4:3,6-dianhydrosorbitol 2,5-dinitrate
71. Iso-puren
72. 1,4:3,6-dianhydro-d-glucitol Dinitrate
73. Sorate-5
74. Sorate-10
75. Isomannide-dinitrate
76. C01da08
77. Nsc-80038
78. Isomak R
79. Cardonit 40
80. Chebi:6061
81. Sst-101
82. Dignionitrat
83. Angidil
84. Diniket
85. Frandol
86. Laserdil
87. Sorbidi Nitras
88. Eurecor
89. Dilatrate Sr
90. (3r,3as,6s,6as)-6-(nitrooxy)-hexahydrofuro[3,2-b]furan-3-yl Nitrate
91. Isomannide Dinitrate
92. Isosorbidi Nitras
93. Nsc80038
94. Iso-mack
95. D-isosorbide Dinitrate-lactose Mixture
96. Ia7306519n
97. Isoket Retard 40
98. Isd
99. Isoket Retard 120
100. Dsstox_cid_25832
101. Dsstox_rid_81160
102. Dsstox_gsid_45832
103. Isosorbide Dinitrato
104. Isosorbide Dinitrato [dcit]
105. Glucitol, 1,4:3,6-dianhydro-, Dinitrate, D-
106. Cedocard Retard
107. Isosorbidi Dinitras [inn-latin]
108. Tyb 3215
109. Diluted Isosorbide Dinitrate
110. Dinitrate D'isosorbide [inn-french]
111. Sdm No. 40
112. Sdm No. 50
113. Smr000857256
114. Isordil (tn)
115. Dinitrato De Isosorbida [inn-spanish]
116. Dilatrate-sr (tn)
117. Ccris 1910
118. Hsdb 3417
119. Sr-05000001658
120. Einecs 201-740-9
121. Nsc 80038
122. Un2907
123. (3r,3as,6s,6as)-hexahydrofuro[3,2-b]furan-3,6-diyl Dinitrate
124. Unii-ia7306519n
125. Cas-87-33-2
126. Prestwick_81
127. 1,4:3,6-dianhydro-2,5-di-o-nitro-d-glucitol
128. Ncgc00094703-01
129. Isosorbide-dinitrate
130. [(3s,3as,6r,6as)-3-nitrooxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-6-yl] Nitrate
131. 1246815-45-1
132. Spectrum_000163
133. 1,4:3, 6-dianhydro-d-glucitol Dinitrate
134. Prestwick0_000714
135. Prestwick1_000714
136. Prestwick2_000714
137. Prestwick3_000714
138. Spectrum2_001069
139. Spectrum3_000600
140. Spectrum4_000025
141. Spectrum5_001057
142. 1,4:3,6-dianhydrosorbitol 2, 5-dinitrate
143. Isosorbide Dinitrate,(s)
144. Isosorbidi Dinitras Dilutes
145. Isosorbidi Dinitras Dilutus
146. Diluted-isosorbide Dinitrate
147. Ec 201-740-9
148. Isosorbide Dinitrate [usan:usp:inn:ban:jan]
149. Schembl8253
150. Chembl6622
151. Bspbio_000927
152. Bspbio_002080
153. Isosorbide Dinitrate, Diluted
154. Kbiogr_000429
155. Kbioss_000643
156. Mls001333561
157. Mls001333562
158. Divk1c_000436
159. Spectrum1500358
160. Isosorbide Dinitrate, Diluted-
161. Spbio_001058
162. Spbio_002848
163. Diluted Isosorbide Dinitrate Rs
164. Bpbio1_001021
165. Gtpl7051
166. Dtxsid0045832
167. Hms501f18
168. Kbio1_000436
169. Kbio2_000643
170. Kbio2_003211
171. Kbio2_005779
172. Kbio3_001580
173. Isosorbide Dinitrate [mi]
174. Ninds_000436
175. Hms1570o09
176. Hms1920h15
177. Hms2091p05
178. Hms2097o09
179. Hms2230c09
180. Hms3714o09
181. Isosorbide Dinitrate [inn]
182. Isosorbide Dinitrate [jan]
183. Pharmakon1600-01500358
184. Isosorbide Dinitrate [hsdb]
185. Isosorbide Dinitrate [usan]
186. Hy-b1409
187. 1,6-dianhydro-d-glucitol Dinitrate
188. Isosorbide Dinitrate [vandf]
189. Tox21_111317
190. Ccg-40110
191. Isosorbide Dinitrate [mart.]
192. Mfcd00868238
193. Nsc757080
194. Zinc18089317
195. 1,6-dianhydrosorbitol 2,5-dinitrate
196. Isosorbide Dinitrate [who-dd]
197. Akos015895227
198. Akos015960761
199. Akos015994784
200. Tox21_111317_1
201. Db00883
202. Gs-6631
203. Isosorbide Dinitrate (jp17/usp/inn)
204. Nsc-757080
205. Idi1_000436
206. Ncgc00178830-01
207. Ncgc00178830-02
208. Ncgc00178830-05
209. Ac-12153
210. D-glucitol,4:3,6-dianhydro-, Dinitrate
211. Isosorbide Dinitrate [orange Book]
212. Bidil Component Isosorbide Dinitrate
213. Isosorbide Dinitrate [usp Impurity]
214. Sbi-0051421.p003
215. Ab00513900
216. Cs-0013129
217. Diluted-isosorbide Dinitrate [who-ip]
218. Glucitol,4:3,6-dianhydro-, Dinitrate, D-
219. Isosorbide Dinitrate Component Of Bidil
220. C07456
221. D00516
222. H10018
223. Ab00052026_07
224. Glucitol, 1,4:3, 6-dianhydro-, Dinitrate, D-
225. Isosorbidi Dinitras Dilutus [who-ip Latin]
226. Q179748
227. 3,6-dianhydro-d-glucitol Dinitrate ; Dilatrate-sr
228. Sr-05000001658-1
229. Sr-05000001658-3
230. Sr-05000001658-5
231. W-104033
232. Z2433066075
233. Isosorbide Mononitrate, Diluted Impurity B [ep Impurity]
234. (3s,3as,6r,6as)-6-(nitrooxy)-hexahydrofuro[3,2-b]furan-3-yl Nitrate
235. [(3r,3as,6s,6as)-3-nitrooxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-6-yl] Nitrate
236. [(3s,3as,6r,6as)-6-nitrooxy-2,3,3a,5,6,6a-hexahydrofuro[2,3-d]furan-3-yl] Nitrate
237. 54650-95-2
238. Isosorbide Dinitrate Mixture With Not <60% Lactose, Mannose, Starch Or Calcium Hydrogen Phosphate
239. Isosorbide Dinitrate Mixture With Not <60% Lactose, Mannose, Starch Or Calcium Hydrogen Phosphate [un2907] [flammable Solid]
| Molecular Weight | 236.14 g/mol |
|---|---|
| Molecular Formula | C6H8N2O8 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 236.02806522 g/mol |
| Monoisotopic Mass | 236.02806522 g/mol |
| Topological Polar Surface Area | 129 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 268 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Bidil |
| PubMed Health | Isosorbide Dinitrate (By mouth) |
| Drug Classes | Antianginal, Coronary Vasodilator |
| Active Ingredient | Hydralazine hydrochloride; isosorbide dinitrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 37.5mg; 20mg |
| Market Status | Prescription |
| Company | Arbor Pharms |
| 2 of 8 | |
|---|---|
| Drug Name | Dilatrate-sr |
| PubMed Health | Isosorbide Dinitrate (By mouth) |
| Drug Classes | Antianginal, Coronary Vasodilator |
| Drug Label | Isosorbide dinitrate (ISDN) is 1,4:3,6-dianhydro-D-glucitol 2,5 dinitrate, an organic nitrate whose structural formula isand whose molecular weight is 236.14. The organic nitrates are vasodilators, active on both arteries and veins. Each dilatrate-... |
| Active Ingredient | Isosorbide dinitrate |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 40mg |
| Market Status | Prescription |
| Company | Auxilium Pharms |
| 3 of 8 | |
|---|---|
| Drug Name | Isordil |
| Drug Label | Isosorbide dinitrate (ISDN) is 1,4:3,6-dianhydro-D-glucitol 2,5-dinitrate, an organic nitrate whose structural formula isand whose molecular weight is 236.14. The organic nitrates are vasodilators, active on both arteries and veins.Isosorbide dinitra... |
| Active Ingredient | Isosorbide dinitrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 30mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 4 of 8 | |
|---|---|
| Drug Name | Isosorbide dinitrate |
| Drug Label | Isosorbide dinitrate, an organic nitrate, is a vasodilator with effects on both arteries and veins. The chemical name for isosorbide dinitrate is 1,4:3,6-dianhydro-D-glucitol dinitrate and the compound has the following structural formula:C6H8N2O8 M.... |
| Active Ingredient | Isosorbide dinitrate |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 30mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Corepharma; Hikma Intl Pharms; Sun Pharm Inds; Sandoz; Par Pharm |
| 5 of 8 | |
|---|---|
| Drug Name | Bidil |
| PubMed Health | Isosorbide Dinitrate (By mouth) |
| Drug Classes | Antianginal, Coronary Vasodilator |
| Active Ingredient | Hydralazine hydrochloride; isosorbide dinitrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 37.5mg; 20mg |
| Market Status | Prescription |
| Company | Arbor Pharms |
| 6 of 8 | |
|---|---|
| Drug Name | Dilatrate-sr |
| PubMed Health | Isosorbide Dinitrate (By mouth) |
| Drug Classes | Antianginal, Coronary Vasodilator |
| Drug Label | Isosorbide dinitrate (ISDN) is 1,4:3,6-dianhydro-D-glucitol 2,5 dinitrate, an organic nitrate whose structural formula isand whose molecular weight is 236.14. The organic nitrates are vasodilators, active on both arteries and veins. Each dilatrate-... |
| Active Ingredient | Isosorbide dinitrate |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 40mg |
| Market Status | Prescription |
| Company | Auxilium Pharms |
| 7 of 8 | |
|---|---|
| Drug Name | Isordil |
| Drug Label | Isosorbide dinitrate (ISDN) is 1,4:3,6-dianhydro-D-glucitol 2,5-dinitrate, an organic nitrate whose structural formula isand whose molecular weight is 236.14. The organic nitrates are vasodilators, active on both arteries and veins.Isosorbide dinitra... |
| Active Ingredient | Isosorbide dinitrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 30mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 8 of 8 | |
|---|---|
| Drug Name | Isosorbide dinitrate |
| Drug Label | Isosorbide dinitrate, an organic nitrate, is a vasodilator with effects on both arteries and veins. The chemical name for isosorbide dinitrate is 1,4:3,6-dianhydro-D-glucitol dinitrate and the compound has the following structural formula:C6H8N2O8 M.... |
| Active Ingredient | Isosorbide dinitrate |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 30mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Corepharma; Hikma Intl Pharms; Sun Pharm Inds; Sandoz; Par Pharm |
Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
In a limited number of patients with diffuse esophageal spasm without gastroesophageal reflux, isosorbide dinitrate has been used effectively to relieve pain, dysphagia and spasm.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1880
Isosorbide dinitrate (in combination with cardiac glycosides and diuretics or with hydralazine) has been used effectively for the treatment of congestive heart failure or other low cardiac output states.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1880
...Isosorbide dinitrate shares the actions of the other nitrates and nitrites. The drug is used for the acute relief of angina pectoris, for prophylactic management in situations likely to provoke angina attacks, and for long term prophylactic management of angina pectoris.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1880
For more Therapeutic Uses (Complete) data for ISOSORBIDE DINITRATE (9 total), please visit the HSDB record page.
...Should be given cautiously in pt with glaucoma.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 788
Dependence and tolerance may occur during chronic use... These possible consequences, as well as large individual variations in clinical response indicates that long-term therapy with high-dose isosorbide dinitrate requires considerable caution.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 321
Drug rash is occasionally produced by all organic nitrates... /organic nitrates/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 850
Most frequent complaint by users...is headache ... also paradoxical increase in anginal pain. Mild gastrointestinal disturbances as well as vertigo and other signs of orthostatic hypotension may occur.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 788
For more Drug Warnings (Complete) data for ISOSORBIDE DINITRATE (10 total), please visit the HSDB record page.
For the prevention of angina pectoris due to coronary artery disease.
Isosorbide Dinitrate is a moderate to long acting oral organic nitrate used for the relief and prophylactic management of angina pectoris. It relaxes the vascular smooth muscle and consequent dilatation of peripheral arteries and veins, especially the latter. Dilatation of the veins promotes peripheral pooling of blood and decreases venous return to the heart, thereby reducing left ventricular end- diastolic pressure and pulmonary capillary wedge pressure (preload). Arteriolar relaxation reduces systemic vascular resistance, systolic arterial pressure, and mean arterial pressure.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Nitric Oxide Donors
A diverse group of agents, with unique chemical structures and biochemical requirements, which generate NITRIC OXIDE. These compounds have been used in the treatment of cardiovascular diseases and the management of acute myocardial infarction, acute and chronic congestive heart failure, and surgical control of blood pressure. (Adv Pharmacol 1995;34:361-81) (See all compounds classified as Nitric Oxide Donors.)
C01DA08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C01 - Cardiac therapy
C01D - Vasodilators used in cardiac diseases
C01DA - Organic nitrates
C01DA08 - Isosorbide dinitrate
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AE - Muscle relaxants
C05AE02 - Isosorbide dinitrate
Absorption
Absorption of isosorbide dinitrate after oral dosing is nearly complete, but bioavailability is highly variable (10% to 90%), with extensive first-pass metabolism in the liver. The average bioavailability of isosorbide dinitrate is about 25%.
Volume of Distribution
2 to 4 L/kg
After sublingual admin, onset of effect is 2-3 min & offset of effect is about 2 hr.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 788
Following sublingual doses of 5 mg, oral conventional tablets of 5 mg, and oral sustained-release tablets of 20 mg, mean peak isosorbide dinitrate levels of 8.9, 3.1, and 1.4 ng/ml, occurred at 30, 40, and 40 min respectively. Plasma levels...after sustained-release dosage...maintained above half of mean peak level for 10 hours.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 5: A Review of the Literature Published during 1976 and 1977. London: The Chemical Society, 1979., p. 40
Chronic oral administration of isosorbide dinitrate (120 to 720 mg daily) has resulted in persistence of parent compound and higher concentration of metabolite in plasma.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 852
Since denitration markedly reduces activity of organic nitrates, their rapid clearance from blood indicates that transient duration of action under these conditions correlates with concentration of parent compounds. /organic nitrates/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 849
For more Absorption, Distribution and Excretion (Complete) data for ISOSORBIDE DINITRATE (13 total), please visit the HSDB record page.
Hepatic
After intravenous or oral admin, primary metabolite in plasma is 5-isosorbide mononitrate.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 849
Major route of metabolism of isosorbide dinitrate in man is also by enzymatic denitration followed by formation of glucuronides.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 849
Biotransformation of organic nitrates is result of reductive hydrolysis catalyzed by hepatic enzyme glutathione-organic nitrate reductase. Enzyme converts lipid-soluble organic nitrate esters into more water-soluble denitrated metabolites & inorganic nitrite. /organic nitrates/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 848
...Biotransformation of isosorbide dinitrate in dogs and in man caused de-esterification. Isosorbide is major urinary metabolite, together with very small amount of the 2- and 5-mononitrates. /isosorbide dinitrate/ was absent from urine of dogs & man.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 137
For more Metabolism/Metabolites (Complete) data for ISOSORBIDE DINITRATE (8 total), please visit the HSDB record page.
1 hour
Half-life 0.7 hours (0.6-2.0; clearance may be decreased and half-life prolonged after chronic dosing.) /from table/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1971
The mean plasma elimination half-life of ISMN is approx 5 hr. /Isosorbide mononitrate/
Medical Economics Co; Physicians Desk Reference 56th ed p.1827 (2002)
After sublingual admin... biological half-life is about 8 hr... .
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 788
Biological half-life is about 8 hr; after oral admin, onset is about 30 min & offset 4-6 hr.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 788
Isosorbide dinitrate is converted to the active nitric oxide to activate guanylate cyclase. This activation increases levels of cyclic guanosine 3',5'-monophosphate (cGMP). cGMP activates protein kinases and causes a series of phosphorylation reactions which leads to dephosphorylation of myosin light chains of smooth muscle fibres. Finally there is a release of calcium ions which causes smooth muscle relaxation and vasodilation.
...Nitrates...are capable of activating guanylate cyclase and causing marked increase in concentration of cyclic guanosine 3',5'-monophosphate (cyclic GMP) in most tissues. This effect is apparently due to action of nitric oxide...nitric acid probably activates the enzyme directly. /organic nitrates/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 848
Isosorbide dinitrate inhibited platelet aggregation with collagen, epinephrine, arachidonate, and ionophore, and blocked both primary and secondary aggregation in response to ADP.
SCHAFER AI ET AL; BLOOD 55(4) 649 (1980)
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2663
Submission : 1976-05-07
Status : Active
Type : II

Registration Number : 217MF11278
Registrant's Address : Hembrunnstr. 17, CH-5605 Dottikon, Switzerland
Initial Date of Registration : 2005-12-20
Latest Date of Registration : --

NDC Package Code : 54864-106
Start Marketing Date : 1983-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (100kg/100kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Kolon Pharmaceutical Co., Ltd.
Registration Date : 2021-03-25
Registration Number : 20200828-209-J-668(1)
Manufacturer Name : Dottikon Exclusive Synthesis AG
Manufacturer Address : Hembrunnstrasse 17 CH-5605 Dottikon Switzerland


GDUFA
DMF Review : Reviewed
Rev. Date : 2024-03-12
Pay. Date : 2024-01-31
DMF Number : 36099
Submission : 2021-12-05
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2016-06-15
Pay. Date : 2016-02-25
DMF Number : 3725
Submission : 1980-02-07
Status : Active
Type : II

Certificate Number : R1-CEP 2013-295 - Rev 00
Issue Date : 2022-02-09
Type : Chemical
Substance Number : 1117
Status : Valid

NDC Package Code : 46438-0001
Start Marketing Date : 2005-08-29
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Erison Pharmaceutical Co., Ltd.
Registration Date : 2023-01-06
Registration Number : 20220825-209-J-1350(1)
Manufacturer Name : Dipharma Francis Srl
Manufacturer Address : Via XXIV Maggio 40-33036 Mereto di Tomba (UD) Italy


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9228
Submission : 1991-07-11
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 345
Submission : 1959-09-10
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5460
Submission : 1984-07-18
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2713
Submission : 1976-07-22
Status : Inactive
Type : II

USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 1021
Submission : 1967-01-12
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7707
Submission : 1988-10-12
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4172
Submission : 1981-05-21
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?