
Synopsis
Synopsis
0
EU WC
0
KDMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
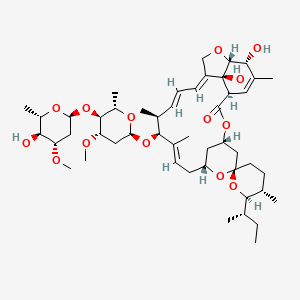

1. Eqvalan
2. Ivermectin
3. Ivomec
4. Mectizan
5. Mk 933
6. Mk-933
7. Mk933
8. Stromectol
1. Ivermectin
2. Dihydroavermectin B1a
3. 70288-86-7
4. 22,23-dihydroavermectin B1a
5. 70161-11-4
6. Ivermectin Component B1a
7. 71827-03-7
8. Avermectin H2b1a
9. 5-o-demethyl-22,23-dihydroavermectin A1a
10. Chebi:63941
11. 91y2202ouw
12. (2ae,4e,5's,6s,6'r,7s,8e,11r,13r,15s,17ar,20r,20ar,20bs)-6'-[(2s)-butan-2-yl]-20,20b-dihydroxy-5',6,8,19-tetramethyl-17-oxo-3',4',5',6,6',10,11,14,15,17,17a,20,20a,20b-tetradecahydro-2h,7h-spiro[11,15-methanofuro[4,3,2-pq][2,6]benzodioxacyclooctadecine-13,2'-pyran]-7-yl 2,6-dideoxy-4-o-(2,6-dideoxy-3-o-methyl-alpha-l-arabino-hexopyranosyl)-3-o-methyl-alpha-l-arabino-hexopyranoside
13. Mk-933
14. (1r,4s,5's,6r,6'r,8r,10e,12s,13s,14e,16e,20r,21r,24s)-6'-[(2s)-butan-2-yl]-21,24-dihydroxy-12-[(2r,4s,5s,6s)-5-[(2s,4s,5s,6s)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-4-methoxy-6-methyloxan-2-yl]oxy-5',11,13,22-tetramethylspiro[3,7,19-trioxatetracyclo[15.6.1.14,8.020,24]pentacosa-10,14,16,22-tetraene-6,2'-oxane]-2-one
15. Ivermectin B1a-d2
16. C48h74o14
17. Unii-91y2202ouw
18. 22,23-dihydroavermectin B(1)a
19. Ncgc00163233-01
20. Ivermectin (ivm)
21. Ivm
22. Einecs 276-046-2
23. Dihydro Avermectin Bla
24. Brn 4643153
25. 22,23-dihydro-5-o-demethylavermectin A1a
26. H2b1a
27. Prestwick3_000156
28. Dsstox_cid_3181
29. Ivermectin (mk-0933)
30. Dsstox_rid_76909
31. Dsstox_gsid_23181
32. Bspbio_000292
33. Schembl312795
34. Bpbio1_000322
35. Chembl263291
36. Dtxsid8023181
37. Chebi:94551
38. Hms2089m09
39. Hms2095o14
40. Hms3712o14
41. Wca82703
42. Tox21_112034
43. Bdbm50409816
44. Mfcd30496678
45. S1351
46. Akos027470116
47. Ivermectin Component B1a [mi]
48. Zinc238808778
49. Zinc252286706
50. Ac-6014
51. Ccg-220156
52. Ncgc00186639-01
53. Ncgc00186639-03
54. As-14167
55. Bi166167
56. Cas-71827-03-7
57. Hy-126937
58. Ab00513813
59. Cs-0108408
60. 22,23-dihydroavermectin B1a; Ivermectin
61. Ab00513813-02
62. Ab00513813-03
63. Ab00513813_04
64. Avermectin A1a, 22,23-dihydro-5-o-demethyl-
65. 288i867
66. Ivermectin, Antibiotic For Culture Media Use Only
67. Q-201262
68. Brd-k24652731-001-02-7
69. Brd-k85554912-001-08-9
70. Q27132923
71. Ivermectin, British Pharmacopoeia (bp) Reference Standard
72. Ivermectin, European Pharmacopoeia (ep) Reference Standard
73. Ivermectin, United States Pharmacopeia (usp) Reference Standard
74. Ivermectin, Pharmaceutical Secondary Standard; Certified Reference Material
75. (2ae,4e,5's,6s,6'r,7s,8e,11r,13r,15s,17ar,20r,20ar,20bs)-6'-[(2s)-butan-2-yl]-20,20b-dihydroxy-5',6,8,19-tetramethyl-17
76. Clooctadecine-13,2'-pyran]-7-yl 2,6-dideoxy-4-o-(2,6-dideoxy-3-o-methyl-alpha-l-arabino-hexopyranosyl)-3-o-methyl-alpha-l-arabino-hexopyranoside
| Molecular Weight | 875.1 g/mol |
|---|---|
| Molecular Formula | C48H74O14 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 8 |
| Exact Mass | 874.50785703 g/mol |
| Monoisotopic Mass | 874.50785703 g/mol |
| Topological Polar Surface Area | 170 Ų |
| Heavy Atom Count | 62 |
| Formal Charge | 0 |
| Complexity | 1680 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 20 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of rosacea
Antiparasitic Agents
Drugs used to treat or prevent parasitic infections. (See all compounds classified as Antiparasitic Agents.)
Insecticides
Pesticides designed to control insects that are harmful to man. The insects may be directly harmful, as those acting as disease vectors, or indirectly harmful, as destroyers of crops, food products, or textile fabrics. (See all compounds classified as Insecticides.)
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AX - Other dermatologicals
D11AX22 - Ivermectin
P - Antiparasitic products, insecticides and repellents
P02 - Anthelmintics
P02C - Antinematodal agents
P02CF - Avermectines
P02CF01 - Ivermectin
 Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
Centrient is a leading manufacturer of Beta-Lactam Antibiotics and a provider of next-generation Statins and Antifungals.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.

GDUFA
DMF Review : Reviewed
Rev. Date : 2014-02-08
Pay. Date : 2012-12-18
DMF Number : 21624
Submission : 2008-05-08
Status : Active
Type : II

Certificate Number : R1-CEP 1999-176 - Rev 05
Issue Date : 2021-03-31
Type : Chemical
Substance Number : 1336
Status : Valid

Registration Number : 304MF10048
Registrant's Address : 46 Waisha Road, Jiaojiang District, Taizhou City, Zhejiang Province, P. R. China 318000
Initial Date of Registration : 2022-03-02
Latest Date of Registration : --

NDC Package Code : 58623-0047
Start Marketing Date : 2014-10-24
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

VMF Number : 5553
Submission : 1995-12-14
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2022-04-06
Pay. Date : 2022-01-13
DMF Number : 36494
Submission : 2021-11-15
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2013-01-09
Pay. Date : 2012-12-14
DMF Number : 21395
Submission : 2008-03-05
Status : Active
Type : II

Certificate Number : R1-CEP 1998-138 - Rev 05
Issue Date : 2020-02-28
Type : Chemical
Substance Number : 1336
Status : Valid

Registration Number : 304MF10006
Registrant's Address : Estrada Coronel Nicolau de Mesquita Taipa, Macau S. A. R. China
Initial Date of Registration : 2022-01-11
Latest Date of Registration : --

NDC Package Code : 55018-121
Start Marketing Date : 1998-07-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17673
Submission : 2004-07-18
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17673
Submission : 2004-07-18
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-01-09
Pay. Date : 2012-12-14
DMF Number : 21395
Submission : 2008-03-05
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2022-04-06
Pay. Date : 2022-01-13
DMF Number : 36494
Submission : 2021-11-15
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-02-08
Pay. Date : 2012-12-18
DMF Number : 21624
Submission : 2008-05-08
Status : Active
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38553
Submission : 2023-06-27
Status : Active
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13037
Submission : 1998-06-15
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 12755
Submission : 1997-11-21
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : IVERMECTIN
Dosage Form : LOTION;TOPICAL
Dosage Strength : 0.5%
Approval Date : 2020-05-06
Application Number : 210720
RX/OTC/DISCN : OTC
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : SKLICE
Dosage Form : LOTION;TOPICAL
Dosage Strength : 0.5%
Approval Date : 2012-02-07
Application Number : 202736
RX/OTC/DISCN : OTC
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : IVERMECTIN
Dosage Form : TABLET;ORAL
Dosage Strength : 3MG
Approval Date : 2014-10-24
Application Number : 204154
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
Brand Name : SOOLANTRA
Dosage Form : CREAM;TOPICAL
Dosage Strength : 1%
Approval Date : 2014-12-19
Application Number : 206255
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : STROMECTOL
Dosage Form : TABLET;ORAL
Dosage Strength : 6MG **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 1996-11-22
Application Number : 50742
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
Brand Name : STROMECTOL
Dosage Form : TABLET;ORAL
Dosage Strength : 3MG
Approval Date : 1998-10-08
Application Number : 50742
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : IVERMECTIN
Dosage Form : CREAM;TOPICAL
Dosage Strength : 1%
Approval Date : 2020-04-13
Application Number : 210225
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : IVERMECTIN
Dosage Form : TABLET;ORAL
Dosage Strength : 3MG
Approval Date :
Application Number : 218324
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : IVERMECTIN
Dosage Form : CREAM;TOPICAL
Dosage Strength : 1%
Approval Date : 2019-09-13
Application Number : 210019
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : IVERMECTIN
Dosage Form : LOTION;TOPICAL
Dosage Strength : 0.5%
Approval Date : 2022-03-21
Application Number : 212485
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Scatol
Dosage Form : Tablet
Dosage Strength : mg
Packaging : Blisterpakning 4item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Scatol
Dosage Form : Tablet
Dosage Strength : mg
Packaging : Blisterpakning 12item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Soolantra
Dosage Form : CREAM
Dosage Strength : 10 MG / G
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Soolantra
Dosage Form : Cream
Dosage Strength : 10 mg/g
Packaging : Tube of plastic
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Soolantra
Dosage Form : Cream
Dosage Strength : 10mg/g
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Ivermectin Medical Valley
Dosage Form : Tablet
Dosage Strength : mg
Packaging : Blisterpakning 4item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Ivermectin Medical Valley
Dosage Form : Tablet
Dosage Strength : mg
Packaging : Blisterpakning 10item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Ivermectin Orifarm
Dosage Form : Tablet
Dosage Strength : mg
Packaging : Blisterpakning 4item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Ivermectin STADA
Dosage Form : Tablet
Dosage Strength : mg
Packaging : Blisterpakning 4item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 1%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 1%
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Granules
Dosage Strength : 12MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Granules
Dosage Strength : 12MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generics
Registration Country : Costa Rica
Brand Name : ivermectin CHEMO®
Dosage Form : TABLET
Dosage Strength : 6MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generics
Registration Country : Costa Rica

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generics
Dosage : TABLET
Dosage Strength : 6MG
Brand Name : ivermectin CHEMO®
Approval Date :
Application Number :
Registration Country : Costa Rica

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 12MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 12MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 6MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 6MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Uncoated Tablet
Dosage Strength : 6mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Uncoated Tablet
Dosage Strength : 6mg
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Uncoated Tablet
Dosage Strength : 12mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Uncoated Tablet
Dosage Strength : 12mg
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Canimect
Dosage Form : Oral Solution
Dosage Strength : 0.1%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Oral Solution
Dosage Strength : 0.1%
Brand Name : Canimect
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : China
Brand Name : Evermec LA
Dosage Form : Liquid Injection
Dosage Strength : 3.15%
Packaging : 100ML,500ML
Approval Date :
Application Number :
Regulatory Info :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 100ML,500ML
Regulatory Info :
Dosage : Liquid Injection
Dosage Strength : 3.15%
Brand Name : Evermec LA
Approval Date :
Application Number :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : China
Brand Name : Evermec-110 Plus
Dosage Form : Liquid Injection
Dosage Strength : 1%; 10%
Packaging : 50ML,100ML,250ML,500ML
Approval Date :
Application Number :
Regulatory Info :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 50ML,100ML,250ML,500ML
Regulatory Info :
Dosage : Liquid Injection
Dosage Strength : 1%; 10%
Brand Name : Evermec-110 Plus
Approval Date :
Application Number :
Registration Country : China

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?