
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
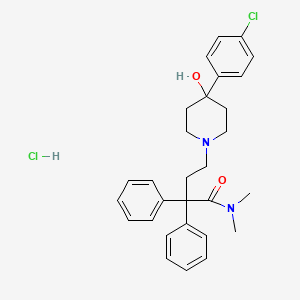

1. Hydrochloride, Loperamide
2. Imodium
3. Loperamide
4. Loperamide Monohydrochloride
5. Monohydrochloride, Loperamide
6. R 18553
7. R-18553
8. R18553
1. 34552-83-5
2. Loperamide Hcl
3. Imodium
4. Suprasec
5. Loperamide (hydrochloride)
6. Fortasec
7. Imodium A-d
8. 4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-n,n-dimethyl-2,2-diphenylbutanamide Hydrochloride
9. Nsc-696356
10. Maalox Anti-diarrheal
11. 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-n,n-dimethyl-2,2-diphenylbutanamide Hydrochloride
12. 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-n,n-dimethyl-2,2-diphenylbutanamide;hydrochloride
13. Mls000069779
14. Chebi:6533
15. 1-piperidinebutanamide, 4-(4-chlorophenyl)-4-hydroxy-n,n-dimethyl-alpha,alpha-diphenyl-, Hydrochloride
16. R 18,553
17. 4-(p-chlorophenyl)-4-hydroxy-n,n-dimethyl-alpha,alpha-diphenyl-1-piperidine Butyramide Hcl
18. 77ti35393c
19. 4-(4-chlorophenyl)-4-hydroxy-n,n-dimethyl-alpha,alpha-diphenylpiperidine-1-butyramide Monohydrochloride
20. 4-(p-chlorophenyl)-4-hydroxy-n,n-dimethyl-alpha,alpha-diphenyl-1-piperidinebutyramide Monohydrochloride
21. Dissenten
22. Lopemid
23. Lopemin
24. Loperyl
25. Smr000058466
26. Imosec
27. Tebloc
28. R-18553
29. Maalox Antidiarrheal
30. Blox
31. Brek
32. Diamode
33. Loseramin
34. Imotil
35. Anti-diarrheal
36. Kao-paverin
37. Diar-aid
38. Kaopectate 1-d
39. K-pek Ii
40. Pepto Diarrhea Control
41. Imodium A-d Ez Chews
42. Pj185
43. Sr-01000075507
44. Up And Up Anti Diarrheal
45. Einecs 252-082-4
46. C29h33cln2o2.hcl
47. Unii-77ti35393c
48. Loperamide, Hcl
49. R 18553
50. Prestwick_302
51. Imodium (tn)
52. Mfcd00058581
53. Cpd000058466
54. Loperamide Hydrochloride [usan:usp:jan]
55. Loperamidi Hydrochloridum
56. Opera_id_1508
57. 4-(4-(p-chlorophenyl)-4-hydroxy-1-piperidyl)-n,n-dimethyl-2,2-diphenylbutyramide Hcl
58. Chembl1707
59. Loperamidehydrochloride
60. Schembl15048
61. R-18553 (hydrochloride)
62. Mls001148627
63. Mls002222200
64. Spectrum2300241
65. Regid_for_cid_71420
66. Loperamide Hydrochloride ,(s)
67. Hy-b0418a
68. Dtxsid00880006
69. Hms1568m10
70. Hms1922j18
71. Pharmakon1600-02300241
72. Bcp28441
73. Loperamide Hydrochloride (jan/usp)
74. Tox21_500708
75. Ccg-39494
76. Loperamide Hydrochloride [mi]
77. Nsc696356
78. Nsc759568
79. S2480
80. Loperamide Hydrochloride [jan]
81. Akos015846351
82. Ac-8242
83. Adl 2-1294
84. Loperamide Hydrochloride [usan]
85. Lp00708
86. Nc00572
87. Nsc 759568
88. Nsc-759568
89. Loperamide Hydrochloride [mart.]
90. Loperamide Hydrochloride [vandf]
91. Loperamide Hydrochloride [usp-rs]
92. Loperamide Hydrochloride [who-dd]
93. Loperamide Hydrochloride [who-ip]
94. Ncgc00094059-01
95. Ncgc00094059-02
96. Ncgc00094059-03
97. Ncgc00094059-04
98. Ncgc00094059-05
99. Ncgc00180886-01
100. Ncgc00180886-02
101. Ncgc00261393-01
102. 1-piperidinebutanamide, 4-(4-chlorophenyl)-4-hydroxy-n,n-dimethyl-.alpha.,.alpha.-diphenyl-, Monohydrochloride
103. 1-piperidinebutanamide, 4-(4-chlorophenyl)-4-hydroxy-n,n-dimethyl-alpha,alpha-diphenyl-, Monohydrochloride
104. 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-n,n-dimethyl-2,2-diphenylbutanamide Hydrochloride
105. As-13181
106. Bl166178
107. B1392
108. Eu-0100708
109. Ft-0627973
110. L0154
111. Sw196602-3
112. Loperamide Hydrochloride [ep Impurity]
113. Loperamide Hydrochloride [orange Book]
114. D00729
115. D78217
116. L 4762
117. Loperamide Hydrochloride [ep Monograph]
118. Loperamide Hydrochloride [usp Monograph]
119. Loperamidi Hydrochloridum [who-ip Latin]
120. 552l835
121. A822272
122. Sr-01000075507-1
123. Sr-01000075507-3
124. Sr-01000075507-8
125. W-106729
126. Q27107231
127. Loperamide Hydrochloride, Vetranal(tm), Analytical Standard
128. Imodium Multi-symptom Relief Component Loperamide Hydrochloride
129. Loperamide Hydrochloride Component Of Imodium Multi-symptom Relief
130. Loperamide Hydrochloride, British Pharmacopoeia (bp) Reference Standard
131. Loperamide Hydrochloride, European Pharmacopoeia (ep) Reference Standard
132. 4-(4-chlorophenyl)-4-hydroxy-n,.alpha.-diphenyl-1-piperidinebutanamide Monohydrochloride
133. 4-(4-chlorophenyl)-4-hydroxy-n,n-dimethyl-?,?-diphenyl-1-piperidinebutanamide Hydrochloride
134. Loperamide Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
135. Loperamide Hydrochloride, United States Pharmacopeia (usp) Reference Standard
136. 4-(4-chlorophenyl)-1-[4-(dimethylamino)-4-oxo-3,3-diphenylbutyl]-4-hydroxypiperidin-1-ium Chloride
137. 4-(4-chlorophenyl)-4-hydroxy-n,n-dimethyl-alpha,alpha-diphenyl-1-piperidinebutanamide Hydrochloride
138. 4-(4-chlorophenyl)-4-hydroxy-n,n-dimethyl-alpha,alpha-diphenyl-1-piperidinebutanamidehydrochloride
139. 4-(p-chlorophenyl)-4-hydroxy-n,n-dimethyl-.alpha.,.alpha.-diphenyl-1-piperidinebutyramide Monohydrochloride
140. 4-[4-(4-chlorophenyl)-4-oxidanyl-piperidin-1-yl]-n,n-dimethyl-2,2-diphenyl-butanamide Hydrochloride
141. Loperamide Hydrochloride For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 513.5 g/mol |
|---|---|
| Molecular Formula | C29H34Cl2N2O2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 7 |
| Exact Mass | 512.1997337 g/mol |
| Monoisotopic Mass | 512.1997337 g/mol |
| Topological Polar Surface Area | 43.8 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 623 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 8 | |
|---|---|
| Drug Name | Imodium |
| Active Ingredient | Loperamide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 2mg |
| Market Status | Prescription |
| Company | Mcneil Cons |
| 2 of 8 | |
|---|---|
| Drug Name | Imodium a-d ez chews |
| Active Ingredient | Loperamide hydrochloride |
| Dosage Form | Tablet, chewable |
| Route | Oral |
| Strength | 2mg |
| Market Status | Over the Counter |
| Company | Mcneil |
| 3 of 8 | |
|---|---|
| Drug Name | Loperamide hydrochloride |
| PubMed Health | Loperamide (By mouth) |
| Drug Classes | Antidiarrheal, Gastrointestinal Agent |
| Drug Label | Loperamide hydrochloride is a white to slightly yellow powder and is freely soluble in methanol, isopropyl alcohol, chloroform and slightly soluble in water.Loperamide hydrochloride, 4-(p-chlorophenyl)-4-hydroxy-N,N-dimethyl-monohydrochloride, is a s... |
| Active Ingredient | Loperamide hydrochloride |
| Dosage Form | Tablet; Capsule; Suspension; Solution |
| Route | Oral |
| Strength | 1mg/5ml; 1mg; 2mg; 1mg/7.5ml |
| Market Status | Over the Counter; Prescription |
| Company | Wockhardt; Ohm Labs; Teva; Lnk; Banner Pharmacaps; Roxane; Perrigo R And D; Hi Tech Pharma; Perrigo; Contract Pharmacal; Mylan |
| 4 of 8 | |
|---|---|
| Drug Name | Loperamide hydrochloride and simethicone |
| Drug Label | IMODIUM (loperamide hydrochloride), 4-(p-chlorophenyl)-4-hydroxy-N,N-dimethyl-a,a-diphenyl-1-piperidinebutyramide monohydrochloride, is a synthetic antidiarrheal for oral use.IMODIUM is available in 2mg capsules.The inactive ingredients are: Lact... |
| Active Ingredient | simethicone; Loperamide hydrochloride |
| Dosage Form | Tablet; Tablet, chewable |
| Route | Oral |
| Strength | 125mg; 2mg |
| Market Status | Over the Counter |
| Company | Ranbaxy; Perrigo |
| 5 of 8 | |
|---|---|
| Drug Name | Imodium |
| Active Ingredient | Loperamide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 2mg |
| Market Status | Prescription |
| Company | Mcneil Cons |
| 6 of 8 | |
|---|---|
| Drug Name | Imodium a-d ez chews |
| Active Ingredient | Loperamide hydrochloride |
| Dosage Form | Tablet, chewable |
| Route | Oral |
| Strength | 2mg |
| Market Status | Over the Counter |
| Company | Mcneil |
| 7 of 8 | |
|---|---|
| Drug Name | Loperamide hydrochloride |
| PubMed Health | Loperamide (By mouth) |
| Drug Classes | Antidiarrheal, Gastrointestinal Agent |
| Drug Label | Loperamide hydrochloride is a white to slightly yellow powder and is freely soluble in methanol, isopropyl alcohol, chloroform and slightly soluble in water.Loperamide hydrochloride, 4-(p-chlorophenyl)-4-hydroxy-N,N-dimethyl-monohydrochloride, is a s... |
| Active Ingredient | Loperamide hydrochloride |
| Dosage Form | Tablet; Capsule; Suspension; Solution |
| Route | Oral |
| Strength | 1mg/5ml; 1mg; 2mg; 1mg/7.5ml |
| Market Status | Over the Counter; Prescription |
| Company | Wockhardt; Ohm Labs; Teva; Lnk; Banner Pharmacaps; Roxane; Perrigo R And D; Hi Tech Pharma; Perrigo; Contract Pharmacal; Mylan |
| 8 of 8 | |
|---|---|
| Drug Name | Loperamide hydrochloride and simethicone |
| Drug Label | IMODIUM (loperamide hydrochloride), 4-(p-chlorophenyl)-4-hydroxy-N,N-dimethyl-a,a-diphenyl-1-piperidinebutyramide monohydrochloride, is a synthetic antidiarrheal for oral use.IMODIUM is available in 2mg capsules.The inactive ingredients are: Lact... |
| Active Ingredient | simethicone; Loperamide hydrochloride |
| Dosage Form | Tablet; Tablet, chewable |
| Route | Oral |
| Strength | 125mg; 2mg |
| Market Status | Over the Counter |
| Company | Ranbaxy; Perrigo |
Antidiarrheals
Miscellaneous agents found useful in the symptomatic treatment of diarrhea. They have no effect on the agent(s) that cause diarrhea, but merely alleviate the condition. (See all compounds classified as Antidiarrheals.)
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 PMC Isochem is your partner for smart CDMOs of Intermediates, APIs, & excipients & a catalog of Intermediates & Generic APIs.
PMC Isochem is your partner for smart CDMOs of Intermediates, APIs, & excipients & a catalog of Intermediates & Generic APIs.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7453
Submission : 1988-04-28
Status : Active
Type : II

NDC Package Code : 49632-070
Start Marketing Date : 2018-07-17
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
 Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.

GDUFA
DMF Review : Reviewed
Rev. Date : 2014-08-20
Pay. Date : 2013-11-25
DMF Number : 27731
Submission : 2013-12-06
Status : Active
Type : II

Certificate Number : CEP 2013-333 - Rev 03
Issue Date : 2024-03-11
Type : Chemical
Substance Number : 929
Status : Valid

Registration Number : 304MF10091
Registrant's Address : 78/A, Bengalrao Nagar, Hyderabad-500 038, Telangana State, India.
Initial Date of Registration : 2022-06-22
Latest Date of Registration : --

Date of Issue : 2022-06-15
Valid Till : 2025-07-14
Written Confirmation Number : WC-0183nA2
Address of the Firm :

NDC Package Code : 66577-015
Start Marketing Date : 2018-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : IMCD Korea Co., Ltd.
Registration Date : 2021-03-10
Registration Number : 20200908-209-J-737(2)
Manufacturer Name : Vasudha Pharma Chem Limited
Manufacturer Address : Unit I, Plot No. 37/A, 38, 39A & B, Phase I IDA, Jeedimetla, Hyderabad-500 055, Telangana, India

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 10942
Submission : 1994-06-17
Status : Inactive
Type : II

Certificate Number : R2-CEP 1994-020 - Rev 05
Issue Date : 2019-11-19
Type : Chemical
Substance Number : 929
Status : Valid

NDC Package Code : 12578-409
Start Marketing Date : 2000-03-27
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Certificate Number : R1-CEP 1996-088 - Rev 00
Issue Date : 2001-12-03
Type : Chemical
Substance Number : 929
Status : Withdrawn by Holder

NDC Package Code : 12578-409
Start Marketing Date : 2000-03-27
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
21 Nov 2024
Reply
16 Sep 2024
Reply
30 Apr 2024
Reply
24 Jan 2024
Reply
23 Mar 2023
Reply
10 Nov 2022
Reply
04 Apr 2022
Reply
21 Mar 2022
Reply
07 Aug 2021
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?