
Synopsis
0
JDMF
0
EU WC
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
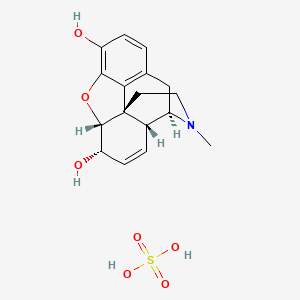

1. Chloride, Morphine
2. Contin, Ms
3. Duramorph
4. Morphia
5. Morphine
6. Morphine Chloride
7. Morphine Sulfate
8. Morphine Sulfate (2:1), Anhydrous
9. Morphine Sulfate (2:1), Pentahydrate
10. Ms Contin
11. Oramorph Sr
12. Sdz 202 250
13. Sdz 202-250
14. Sdz 202250
15. Sdz202 250
16. Sdz202-250
17. Sdz202250
18. Sulfate, Morphine
1. Morphine Sulfate
2. Schembl29317
3. Morphinesulfatenarcoticanalgesic
| Molecular Weight | 383.4 g/mol |
|---|---|
| Molecular Formula | C17H21NO7S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 383.10387318 g/mol |
| Monoisotopic Mass | 383.10387318 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 576 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Astramorph pf |
| PubMed Health | Morphine (Injection) |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml; 0.5mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 2 of 6 | |
|---|---|
| Drug Name | Duramorph pf |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml; 0.5mg/ml |
| Market Status | Prescription |
| Company | Hikma Maple |
| 3 of 6 | |
|---|---|
| Drug Name | Morphine sulfate |
| PubMed Health | Morphine Sulfate Liposome (Injection) |
| Drug Classes | Analgesic, Anesthetic Adjunct, Central Nervous System Agent |
| Drug Label | DESCRIPTIONChemically, morphine sulfate is 7, 8-didehydro-4, 5-epoxy-17-methylmorphinan-3, 6-diol sulfate (2:1) (salt) pentahydrate... |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Tablet, extended release; Tablet; Injectable; Capsule, extended release; Solution |
| Route | Intramuscular, intravenous; oral; Injection; Oral |
| Strength | 2mg/ml; 200mg; 10mg/5ml; 1mg/ml; 8mg/ml (8mg/ml); 30mg; 10mg/ml (10mg/ml); 100mg/5ml; 90mg; 15mg; 120mg; 4mg/ml (4mg/ml); 10mg/ml; 0.5mg/ml; 2mg/ml (2mg/ml); 75mg; 4mg/ml; 5mg/ml; 100mg; 5mg/ml (5mg/ml); 8mg/ml; 50mg; 60mg; 10mg; 15mg/ml; 20mg/5ml; 80mg; |
| Market Status | Prescription |
| Company | Clonmel Hlthcare; Vintage Pharms; Mylan Pharms; Upsher Smith; Hospira; Mallinckrodt; Rhodes Pharms; Meridian Medcl; Par Pharm; Roxane; Lannett Holdings; Nesher Pharms; Vistapharm; Actavis Elizabeth; Paddock; Caraco; Ranbaxy Labs; Bd Rx |
| 4 of 6 | |
|---|---|
| Drug Name | Astramorph pf |
| PubMed Health | Morphine (Injection) |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml; 0.5mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 5 of 6 | |
|---|---|
| Drug Name | Duramorph pf |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml; 0.5mg/ml |
| Market Status | Prescription |
| Company | Hikma Maple |
| 6 of 6 | |
|---|---|
| Drug Name | Morphine sulfate |
| PubMed Health | Morphine Sulfate Liposome (Injection) |
| Drug Classes | Analgesic, Anesthetic Adjunct, Central Nervous System Agent |
| Drug Label | DESCRIPTIONChemically, morphine sulfate is 7, 8-didehydro-4, 5-epoxy-17-methylmorphinan-3, 6-diol sulfate (2:1) (salt) pentahydrate... |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Tablet, extended release; Tablet; Injectable; Capsule, extended release; Solution |
| Route | Intramuscular, intravenous; oral; Injection; Oral |
| Strength | 2mg/ml; 200mg; 10mg/5ml; 1mg/ml; 8mg/ml (8mg/ml); 30mg; 10mg/ml (10mg/ml); 100mg/5ml; 90mg; 15mg; 120mg; 4mg/ml (4mg/ml); 10mg/ml; 0.5mg/ml; 2mg/ml (2mg/ml); 75mg; 4mg/ml; 5mg/ml; 100mg; 5mg/ml (5mg/ml); 8mg/ml; 50mg; 60mg; 10mg; 15mg/ml; 20mg/5ml; 80mg; |
| Market Status | Prescription |
| Company | Clonmel Hlthcare; Vintage Pharms; Mylan Pharms; Upsher Smith; Hospira; Mallinckrodt; Rhodes Pharms; Meridian Medcl; Par Pharm; Roxane; Lannett Holdings; Nesher Pharms; Vistapharm; Actavis Elizabeth; Paddock; Caraco; Ranbaxy Labs; Bd Rx |
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2014-08-29
Pay. Date : 2014-08-18
DMF Number : 21588
Submission : 2008-04-30
Status : Active
Type : II

Certificate Number : CEP 2001-239 - Rev 07
Issue Date : 2024-06-20
Type : Chemical
Substance Number : 1244
Status : Valid

NDC Package Code : 49812-0149
Start Marketing Date : 2005-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Baeksu Pharmaceutical Co., Ltd.
Registration Date : 2021-07-13
Registration Number : 20190812-209-J-406(2)
Manufacturer Name : Macfarlan Smith Limited
Manufacturer Address : 10 Wheatfield Road, Edinburgh, EH11 2QA, United Kingdom

| Available Reg Filing : ROW |
 Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.

Certificate Number : CEP 2000-126 - Rev 07
Issue Date : 2024-04-26
Type : Chemical
Substance Number : 1244
Status : Valid

| Available Reg Filing : ROW |
 Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-02-05
Pay. Date : 2012-11-23
DMF Number : 6967
Submission : 1987-05-08
Status : Active
Type : II

NDC Package Code : 51634-0969
Start Marketing Date : 2000-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.

GDUFA
DMF Review : Reviewed
Rev. Date : 2016-07-19
Pay. Date : 2015-12-10
DMF Number : 29317
Submission : 2015-11-25
Status : Active
Type : II

NDC Package Code : 51634-0969
Start Marketing Date : 2000-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 14641
Submission : 1999-12-23
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16433
Submission : 2003-02-21
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Regulatory Info :
Registration Country : Iran
Brand Name :
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Iran
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : KADIAN
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 20MG
Packaging :
Approval Date : 1996-07-03
Application Number : 20616
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : KADIAN
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 50MG
Packaging :
Approval Date : 1996-07-03
Application Number : 20616
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : KADIAN
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 100MG
Packaging :
Approval Date : 1996-07-03
Application Number : 20616
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : KADIAN
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 30MG
Packaging :
Approval Date : 2001-03-09
Application Number : 20616
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : KADIAN
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 60MG
Packaging :
Approval Date : 2001-03-09
Application Number : 20616
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : KADIAN
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 200MG
Packaging :
Approval Date : 2007-02-27
Application Number : 20616
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : KADIAN
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Packaging :
Approval Date : 2007-04-20
Application Number : 20616
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : KADIAN
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 40MG
Packaging :
Approval Date : 2012-07-09
Application Number : 20616
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : KADIAN
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 70MG
Packaging :
Approval Date : 2012-07-09
Application Number : 20616
Regulatory Info : DISCN
Registration Country : USA
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Regulatory Info :
Registration Country : Iran
Brand Name :
Dosage Form : Tablet
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Iran
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Iran
 Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Regulatory Info : Dossier Availability: Q3 2024
Registration Country : Poland
Brand Name :
Dosage Form : Solution for Infusion
Dosage Strength : 10MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info : Dossier Availability: Q3 2024
Registration Country : Poland
 Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Packaging :
Regulatory Info : Dossier Availability: Q3 2024
Dosage : Solution for Infusion
Dosage Strength : 10MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : Poland
 Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Regulatory Info : Dossier Availability: Q3 2024
Registration Country : Poland
Brand Name :
Dosage Form : Solution for Infusion
Dosage Strength : 20MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info : Dossier Availability: Q3 2024
Registration Country : Poland
 Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Packaging :
Regulatory Info : Dossier Availability: Q3 2024
Dosage : Solution for Infusion
Dosage Strength : 20MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : Poland
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 10mg
Packaging : Pack Size 10x10
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging : Pack Size 10x10
Regulatory Info :
Dosage : Tablet
Dosage Strength : 10mg
Brand Name :
Approval Date :
Application Number :
Registration Country : India
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 30mg
Packaging : Pack Size 10x10
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging : Pack Size 10x10
Regulatory Info :
Dosage : Tablet
Dosage Strength : 30mg
Brand Name :
Approval Date :
Application Number :
Registration Country : India
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Extended Release Table...
Dosage Strength : 10mg
Packaging : Pack Size 6x10; 10x10
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging : Pack Size 6x10; 10x10
Regulatory Info :
Dosage : Extended Release Table...
Dosage Strength : 10mg
Brand Name :
Approval Date :
Application Number :
Registration Country : India
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Extended Release Table...
Dosage Strength : 30mg
Packaging : Pack Size 10x10
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging : Pack Size 10x10
Regulatory Info :
Dosage : Extended Release Table...
Dosage Strength : 30mg
Brand Name :
Approval Date :
Application Number :
Registration Country : India
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 10mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 10mg/ml
Brand Name :
Approval Date :
Application Number :
Registration Country : India
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 15mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 15mg/ml
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Ethypharm is an international Pharma company with European roots manufacturing and commercializing essential drugs all over the world.
Ethypharm is an international Pharma company with European roots manufacturing and commercializing essential drugs all over the world.
Regulatory Info :
Registration Country : France
Brand Name :
Dosage Form : Oral Dispersible Table...
Dosage Strength : 5MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : France
 Ethypharm is an international Pharma company with European roots manufacturing and commercializing essential drugs all over the world.
Ethypharm is an international Pharma company with European roots manufacturing and commercializing essential drugs all over the world.
Packaging :
Regulatory Info :
Dosage : Oral Dispersible Table...
Dosage Strength : 5MG
Brand Name :
Approval Date :
Application Number :
Registration Country : France
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
18 Nov 2024
Reply
03 May 2024
Reply
02 Apr 2024
Reply
25 Oct 2023
Reply
26 Jul 2023
Reply
17 Jan 2023
Reply
17 Oct 2022
Reply
22 Aug 2022
Reply
16 Aug 2022
Reply
21 Sep 2021
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?