
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
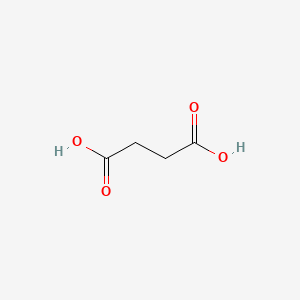

1. 1,2 Ethanedicarboxylic Acid
2. 1,2-ethanedicarboxylic Acid
3. 1,4 Butanedioic Acid
4. 1,4-butanedioic Acid
5. Ammonium Succinate
6. Butanedioic Acid
7. Potassium Succinate
8. Succinate
9. Succinate, Ammonium
10. Succinate, Potassium
1. Butanedioic Acid
2. 110-15-6
3. Amber Acid
4. Wormwood Acid
5. Asuccin
6. Dihydrofumaric Acid
7. Katasuccin
8. 1,2-ethanedicarboxylic Acid
9. Bernsteinsaure
10. Ethylenesuccinic Acid
11. 1,4-butanedioic Acid
12. Wormwood
13. Succinicum Acidum
14. Butandisaeure
15. Acidum Succinicum
16. Butanedionic Acid
17. Kyselina Jantarova
18. Butane Diacid
19. Ethylene Dicarboxylic Acid
20. Spirit Of Amber
21. Bernsteinsaure [german]
22. Kyselina Jantarova [czech]
23. Ammonium Succinate
24. Hsdb 791
25. Succinic-acid
26. Mfcd00002789
27. Succ
28. Nsc 106449
29. Ai3-06297
30. Ab6mnq6j6l
31. Nsc-106449
32. Butanedioic Acid, Homopolymer
33. E363
34. Fema No. 4719
35. Chebi:15741
36. C4-beta-polymorph
37. Nsc25949
38. Succinicacid(industrialgrade&foodgrade)
39. Ncgc00159372-02
40. Ncgc00159372-04
41. Succinellite
42. Acide Succinique
43. Sal Succini
44. Acid Of Amber
45. Dsstox_cid_3602
46. Wln: Qv2vq
47. Dsstox_rid_77102
48. Dsstox_gsid_23602
49. 26776-24-9
50. Sin
51. Ethylene Succinic Acid
52. Ethanedicarboxylic Acid
53. Bernsteinsaeure
54. Sodium Succinate (anhydrous)
55. Succinate, 9
56. Acide Butanedioique
57. Cas-110-15-6
58. Succinic Acid [nf]
59. Succinic Acid (8ci)
60. Butanedioic Acid (9ci)
61. Einecs 203-740-4
62. Unii-ab6mnq6j6l
63. Brn 1754069
64. Dihydrofumarate
65. Succinicate
66. Butanedioic Acid Diammonium Salt
67. Salt Of Amber
68. 1cze
69. Nat.succinic Acid
70. 1,4-butanedioate
71. Succinic Acid, 6
72. Succinic Acid, Fcc
73. Succinic Acide,(s)
74. Succinic Acid (sa)
75. 1,4-butandioic Acid
76. Succinic Acid, 99%
77. Succinic Acid, Natural
78. 4lh2
79. 1,2-ethanedicarboxylate
80. Substrate Analogue, 11
81. Suc
82. Succinic Acid, Acs Grade
83. Bmse000183
84. Bmse000968
85. Chembl576
86. Ec 203-740-4
87. Succinic Acid [ii]
88. Succinic Acid [mi]
89. Hooc-ch2-ch2-cooh
90. Succinic Acid [fcc]
91. A 12084
92. Succinic Acid [hsdb]
93. Succinic Acid [inci]
94. 4-02-00-01908 (beilstein Handbook Reference)
95. Succinic Acid [vandf]
96. Succinic Acid [mart.]
97. Gtpl3637
98. Succinic Acid [usp-rs]
99. Succinic Acid [who-dd]
100. Dtxsid6023602
101. Succinicum Acidum [hpus]
102. Bdbm26121
103. Succinic Acid (butanedioic Acid)
104. Hms3885o04
105. Zinc895030
106. Hy-n0420
107. Str02803
108. Tox21_111612
109. Tox21_201918
110. Tox21_303247
111. Bbl002473
112. Lmfa01170043
113. Nsc-25949
114. Nsc106449
115. S3791
116. Stk387105
117. Succinic Acid [usp Impurity]
118. Succinic Acid, >=99%, Fcc, Fg
119. Succinic Acid, Bioxtra, >=99.0%
120. Akos000118899
121. Tox21_111612_1
122. Ccg-266069
123. Db00139
124. Ncgc00159372-03
125. Ncgc00159372-05
126. Ncgc00159372-06
127. Ncgc00257092-01
128. Ncgc00259467-01
129. Succinic Acid, Acs Reagent, >=99.0%
130. Bp-21128
131. Succinic Acid, Reagentplus(r), >=99.0%
132. Adipic Acid Impurity B [ep Impurity]
133. Cs-0008946
134. Ft-0652509
135. Ft-0773657
136. N1941
137. S0100
138. Succinic Acid 100 Microg/ml In Acetonitrile
139. Succinic Acid, P.a., Acs Reagent, 99.0%
140. Succinic Acid, Saj First Grade, >=99.0%
141. Succinic Acid High Purity Grade 2.5kg
142. Succinic Acid, Purum P.a., >=99.0% (t)
143. Succinic Acid, Saj Special Grade, >=99.5%
144. 1,4-butanedioic Acid (succinic Acid)
145. A14596
146. C00042
147. D85169
148. Succinic Acid, Vetec(tm) Reagent Grade, 98%
149. Ab01332192-02
150. Q213050
151. Sr-01000944556
152. J-002386
153. Sr-01000944556-2
154. Z57127453
155. F2191-0239
156. 37e8fffb-70da-4399-b724-476bd8715ef0
157. Succinic Acid, Certified Reference Material, Tracecert(r)
158. Succinic Acid, Puriss. P.a., Acs Reagent, >=99.5% (t)
159. Succinic Acid, United States Pharmacopeia (usp) Reference Standard
160. Succinic Acid, Matrix Substance For Maldi-ms, >=99.5% (t), Ultra Pure
161. Succinic Acid, Anhydrous, Free-flowing, Redi-dri(tm), Acs Reagent, >=99.0%
162. Succinic Acid, Bioreagent, Suitable For Cell Culture, Suitable For Insect Cell Culture
163. Succinic Acid, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 118.09 g/mol |
|---|---|
| Molecular Formula | C4H6O4 |
| XLogP3 | -0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 118.02660867 g/mol |
| Monoisotopic Mass | 118.02660867 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 92.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPTL THER/ Succinic acid (100 mM) significantly inhibited systemic anaphylaxis induced by compound 48/80 /a potent mast cell degranulator/ in mice and dose-dependently inhibited local anaphylaxis activated by anti-dinitrophenyl IgE. Further 10 and 100 mM significantly inhibited histamine release from rat peritoneal mast cells activated by compound 48/80 or anti-dinitrophenyl IgE. In addition succinic acid (0.1 and 1 mM) had a significant inhibitory effect on anti-dinitrophenyl IgE-induced tumor necrosis factor-alpha secretion from rat peritoneal mast cells. The level of cyclic AMP in rat peritoneal mast cells, when succinic acid (100 mM) was added, transiently and significantly increased about 4 times compared with that of basal cells. These results suggest a possible use of succinic acid in managing mast cell-dependent anaphylaxis.
Kim HM et al;
For nutritional supplementation, also for treating dietary shortage or imbalance
Succinic acid occurs normally in human urine (1.9-8.8 mg/L).
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V5 758
Succinic acid is a normal intermediary metabolite and a constituent of the citric acid cycle. It is readily metabolized when administered to animals, but may be partly excreted unchanged in the urine if large doses are fed.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V5 757
Succinate is an essential component of the Krebs or citric acid cycle and serves an electron donor in the production of fumaric acid and FADH2. It also has been shown to be a good "natural" antibiotic because of its relative acidic or caustic nature (high concentrations can even cause burns). Succinate supplements have been shown to help reduce the effects of hangovers by activating the degradation of acetaldehyde - a toxic byproduct of alcohol metabolism - into CO2 and H2O through aerobic metabolism. Succinic acid has been shown to stimulate neural system recovery and bolster the immune system. Claims have also been made that it boosts awareness, concentration and reflexes.
 Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
About the Company : Faran Shimi Pharmaceutical Company, established in 2001 and affiliated with Golrang Pharmaceutical Investment Co, manufactures high-quality Active Pharmaceutical Ingredients (APIs)...
About the Company : QUALITY CHEMICALS, SL is a fine chemicals manufacturer of pure salts (mineral, metallic, organic, etc.), solvents, acids, basis and some organics. Our products are destined to a wi...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
(9H-fluoren-9-yl)methyl ((2R,3R)-1,3dihydroxybutan...
CAS Number : 176380-53-3
End Use API : Succinic Acid
About The Company : USV is a leading health care company with focus on Active Pharmaceutical Ingredients (marketed globally with emphasis on regulated markets of USA, European Unio...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Dosage Form : Injectable / Parenteral
Grade : Parenteral
Brand Name : Glycerol Multi-Compendial
Application : Parenteral
Excipient Details : A & C's Glycerol multi-compendial is an excipient that meets USP-NF, EP and BP monographs.
Pharmacopoeia Ref : Multi-compendial
Technical Specs : Not Available
Ingredient(s) : Glycerol Excipient
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Application : Parenteral
Excipient Details : A & C's Glycerol is an excipient which meets the USP monograph.
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Brand Name : NaCl Multi-compendial Low...
Application : Parenteral
Excipient Details : A & C's Sodium Chloride multi-compendial low endotoxin is an excipient meeting USP-NF, EP, BP and JP monographs.
Pharmacopoeia Ref : Multi-compendial
Technical Specs : Low Endotoxin
Ingredient(s) : Sodium Chloride Excipient
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Brand Name : Sodium Chloride USP
Application : Parenteral
Excipient Details : A & C's Sodium Chloride is an excipient meeting the USP monograph.
Dosage Form : Syrup
Grade : Oral
Brand Name : Sucrose Multi-Compendial
Application : Taste Masking
Excipient Details : A & C's Sucrose multi-compendial is a pharmaceutical excipient grade meeting the current specifications of USP-NF, EP and JP monographs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Softgel Capsule
Grade : Not Available
Application : Thickeners and Stabilizers
Excipient Details : Structure-building consistency factor with dry feel, forms crystalline barrier on skin
Pharmacopoeia Ref : Ph. Eur., USP-NF, JP: Stearic ...
Technical Specs : Not Available
Ingredient(s) : Stearic Acid
Dosage Form : Tablet
Grade : Not Available
Application : Granulation
Dosage Form : Granule / Pellet
Grade : Not Available
Application : Direct Compression
Excipient Details : Ready-to-use direct compression solution for tablets.
Dosage Form : Granule / Pellet
Grade : Not Available
Application : Direct Compression
Excipient Details : Ready-to-use direct compression solution for lozenges, chewables and effervescent tablets.
Pharmacopoeia Ref : Ph. Eur., USP/NF and J.P
Technical Specs : Not Available
Ingredient(s) : Lactose
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipient Details : Helps to manufacture Oral Dosage and Nutraceutical forms by acting as a filler-binder while serving as a fibre source for your customers.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Tablet
Grade : Not Available
Brand Name : ReadiLYCOAT® D CLEAR 110...
Application : Film Formers & Plasticizers
Excipient Details : A natural inert polymer and ready-to-use coating system for fast aqueous film coating saves up to 50% or more time.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Pea Starch, Sorbitol, Stearic Acid
Dosage Form : Tablet
Grade : Not Available
Brand Name : ReadiLYCOAT® D WHITE 010...
Application : Film Formers & Plasticizers
Excipient Details : A natural inert polymer and ready-to-use coating system for fast aqueous film coating saves up to 50% or more time.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Pea Starch, Sorbitol, Titanium Dioxide, Stearic Acid
Dosage Form : Tablet
Grade : Not Available
Application : Disintegrants & Superdisintegrants
Excipient Details : It is a superdisintegrant that provides an efficient disintegration at low level of use
Dosage Form : Capsule
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipient Details : It is a Co-Processed Lactose starch for filler/binder having strong disintegrant properties.
Dosage Form : Cream / Lotion / Ointment
Grade : Not Available
Application : Thickeners and Stabilizers
Excipient Details : A co-processed product used as a secondary stabilizer and suspension agent in semi-solides formulas.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Colloidal Microcrystalline Cellulose
Dosage Form : Cream / Lotion / Ointment
Grade : Not Available
Application : Thickeners and Stabilizers
Excipient Details : A co-processed product used as a secondary stabilizer and suspension agent in semi-solides formulas.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Colloidal Microcrystalline Cellulose
Dosage Form : Cream / Lotion / Ointment
Grade : Not Available
Application : Thickeners and Stabilizers
Excipient Details : A co-processed product used as a secondary stabilizer and suspension agent in semi-solides formulas.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Colloidal Microcrystalline Cellulose
Dosage Form : Cream / Lotion / Ointment
Grade : Not Available
Brand Name : TABULOSE® SC 591F
Application : Thickeners and Stabilizers
Excipient Details : A co-processed product used as a secondary stabilizer and suspension agent in semi-solides formulas.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Colloidal Microcrystalline Cellulose
Dosage Form : Cream / Lotion / Ointment
Grade : Not Available
Application : Thickeners and Stabilizers
Excipient Details : A co-processed product used as a secondary stabilizer and suspension agent in semi-solides formulas.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Colloidal Microcrystalline Cellulose
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Dosage Form : Capsule, Orodispersible Tablet, Tablet
Grade : Oral
Category : Disintegrants & Superdisintegrants, Fillers, Diluents & Binders
Grade : Oral
Category : Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Lubricants & Glidants
Application : Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Lubricants & Glidants
Excipient Details : ProBlend (Microcrystalline Cellulose) is used as a filler, binder, glidant, DC & co-processed excipient in tablets, capsules, and MUPS formulations.
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Disintegrants & Superdisintegrants
Grade : Oral
Category : Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders
Grade : Oral
Category : Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders, Thickeners and Stabilizers
Grade : Oral
Category : Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders
Brand Name : Microlose™ M60 P60
Application : Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders
Excipient Details : Microlose M60 P60 is used as a filler, binder, directly compressible, and co-processed excipient in tablets and capsules.
Pharmacopoeia Ref : In-house
Technical Specs : Lactose Monohydrate – 40%, Microcrystalline cellulose – 60%
Ingredient(s) : Lactose Monohydrate
Grade : Oral
Category : Fillers, Diluents & Binders, Lubricants & Glidants
Application : Fillers, Diluents & Binders, Lubricants & Glidants
Excipient Details : Most popular excipient for the production of tablets and capsules. Offering an efficient and low dosage in capsules.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-6-10 m2/g; Particle Size-7-11 µm
Ingredient(s) : Magnesium Stearate
Dosage Form : Tablet
Grade : Oral
Category : Direct Compression, Fillers, Diluents & Binders, Granulation
Application : Direct Compression, Fillers, Diluents & Binders, Granulation
Excipient Details : AceCel is suitable for majority of the directly compressible actives, combines good flow and high compressibility.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Grade : Oral
Category : Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Lubricants & Glidants
Application : Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Lubricants & Glidants
Excipient Details : ProBlend (Microcrystalline Cellulose) is used as a filler, binder, glidant, DC & co-processed excipient in tablets, capsules, and MUPS formulations.
Grade : Oral
Category : Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders
Grade : Oral
Category : Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders, Thickeners and Stabilizers
Grade : Oral
Category : Co-Processed Excipients, Controlled & Modified Release, Direct Compression
Grade : Oral
Category : Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders
Brand Name : Microlose™ M60 P60
Application : Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders
Excipient Details : Microlose M60 P60 is used as a filler, binder, directly compressible, and co-processed excipient in tablets and capsules.
Pharmacopoeia Ref : In-house
Technical Specs : Lactose Monohydrate – 40%, Microcrystalline cellulose – 60%
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Oral
Category : Direct Compression, Disintegrants & Superdisintegrants, Granulation
Dosage Form : Tablet
Grade : Oral
Category : Direct Compression, Fillers, Diluents & Binders, Granulation
Application : Direct Compression, Fillers, Diluents & Binders, Granulation
Excipient Details : AceCel is suitable for majority of the directly compressible actives, combines good flow and high compressibility.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Application : Direct Compression
Pharmacopoeia Ref : Conforms to USP-NF, Ph.Eur., J...
Technical Specs : Density- Tapped density- 440 g/l, Bulk density- 310 g/l; Particl...
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Tablet
Grade : Oral
Category : Direct Compression, Fillers, Diluents & Binders, Granulation
Application : Direct Compression, Fillers, Diluents & Binders, Granulation
Excipient Details : HiCel acts as a strong & dry binder. It facilitates low tablet friability & promotes rapid tablet disintegration.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Orodispersible Tablet
Grade : Oral
Category : Direct Compression, Disintegrants & Superdisintegrants
Application : Direct Compression, Disintegrants & Superdisintegrants
Grade : Oral
Category : Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Lubricants & Glidants
Application : Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Lubricants & Glidants
Excipient Details : ProBlend (Microcrystalline Cellulose) is used as a filler, binder, glidant, DC & co-processed excipient in tablets, capsules, and MUPS formulations.
Grade : Oral
Category : Fillers, Diluents & Binders, Lubricants & Glidants
Application : Fillers, Diluents & Binders, Lubricants & Glidants
Excipient Details : Most popular excipient for the production of tablets and capsules. Offering an efficient and low dosage in capsules.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-6-10 m2/g; Particle Size-7-11 µm
Ingredient(s) : Magnesium Stearate
Grade : Oral
Category : Fillers, Diluents & Binders, Lubricants & Glidants
Application : Fillers, Diluents & Binders, Lubricants & Glidants
Excipient Details : Higher specific surface area and a smaller median particle size. This product is preferred for more critical and very fine herbal formulations.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-8-12 m2/g; Particle Size-5-9 µm
Ingredient(s) : Magnesium Stearate
Grade : Oral
Category : Fillers, Diluents & Binders, Lubricants & Glidants
Application : Fillers, Diluents & Binders, Lubricants & Glidants
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-6-8 m2/g; Particle Size-7-11 µm
Ingredient(s) : Magnesium Stearate
Grade : Oral
Category : Fillers, Diluents & Binders, Lubricants & Glidants
Application : Fillers, Diluents & Binders, Lubricants & Glidants
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Not Available
Ingredient(s) : Magnesium Stearate
Brand Name : Magnesium Stearate
Application : Lubricants & Glidants
Excipient Details : Lubricants, Anti-adhesive, Glidant
Dosage Form : Cream / Lotion / Ointment, Tablet
Grade : Oral
Category : Emulsifying Agents, Lubricants & Glidants, Thickeners and Stabilizers
Application : Emulsifying Agents, Lubricants & Glidants, Thickeners and Stabilizers
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Category : Parenteral, Thickeners and Stabilizers
Dosage Form : Cream / Lotion / Ointment, Emulsion, Injectable / Parenteral, Softgels, Tablet
Grade : Oral, Topical, Parenteral
Category : Film Formers & Plasticizers, Parenteral, Topical
Brand Name : Sodium Chloride USP
Application : Parenteral
Excipient Details : A & C's Sodium Chloride is an excipient meeting the USP monograph.
Brand Name : Glycerol Multi-Compendial
Application : Parenteral
Excipient Details : A & C's Glycerol multi-compendial is an excipient that meets USP-NF, EP and BP monographs.
Pharmacopoeia Ref : Multi-compendial
Technical Specs : Not Available
Ingredient(s) : Glycerol Excipient
Application : Parenteral
Excipient Details : A & C's Glycerol is an excipient which meets the USP monograph.
Brand Name : NaCl Multi-compendial Low Endotoxin
Application : Parenteral
Excipient Details : A & C's Sodium Chloride multi-compendial low endotoxin is an excipient meeting USP-NF, EP, BP and JP monographs.
Pharmacopoeia Ref : Multi-compendial
Technical Specs : Low Endotoxin
Ingredient(s) : Sodium Chloride Excipient
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Taste Masking
Application : Chewable & Orodispersible Aids, Taste Masking
Excipient Details : HiCel CE15 offers a superior mouthfeel with less chalkiness and gritness in chewable tablets and orally disintegrating tablets (ODTs).
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Guar Gum Excipient
Dosage Form : Capsule, Emulsion, Solution, Suspension, Syrup, Tablet
Grade : Oral
Category : Coating Systems & Additives, Taste Masking, Thickeners and Stabilizers
Brand Name : Sucrose Multi-Compendial
Application : Coating Systems & Additives, Taste Masking, Thickeners and Stabilizers
Excipient Details : A & C's Sucrose multi-compendial is a pharmaceutical excipient grade meeting the current specifications of USP-NF, EP and JP monographs.
Application : Coating Systems & Additives, Taste Masking
Excipient Details : Ready mix sugar coating system.
Pharmacopoeia Ref : USP, EP, JP & having US DMF
Technical Specs : Sprayable sugar coating system for solid oral dosage form
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Taste Masking
Application : Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Taste Masking
Excipient Details : F-Melt Type C is a pharmaceutical excipient used in oral dosage forms like orally disintegrating tablets, sachets, dispersible tablets, chewable tablets and sublingual tablets.
Pharmacopoeia Ref : Conforms to Japanese Pharmaceu...
Technical Specs : Not Available
Ingredient(s) : Crospovidone
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?