
Synopsis
Synopsis
0
VMF
0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
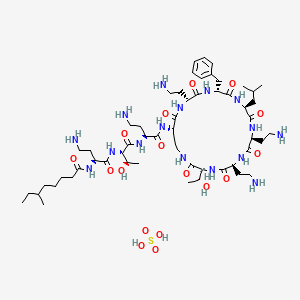

1. Polymyxin B, Sulfate (salt)
2. 1405-20-5
3. Polymixin B Sulphate
4. Ks-1428
5. Pmb
6. Akos025392172
7. Polymyxin B Sulfate, Bacillus Polymyxa
8. M02428
9. Polymyxin B Sulfate (1404-26-8 Free Base)
10. 405p205
| Molecular Weight | 1301.6 g/mol |
|---|---|
| Molecular Formula | C56H100N16O17S |
| Hydrogen Bond Donor Count | 20 |
| Hydrogen Bond Acceptor Count | 22 |
| Rotatable Bond Count | 29 |
| Exact Mass | 1300.71730696 g/mol |
| Monoisotopic Mass | 1300.71730696 g/mol |
| Topological Polar Surface Area | 574 Ų |
| Heavy Atom Count | 90 |
| Formal Charge | 0 |
| Complexity | 2240 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 12 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Polymyxin b sulfate |
| Drug Label | Polymyxin B for Injection, USP is one of a group of basic polypeptide antibiotics derived from B polymyxa (B aerosporous). Polymyxin B sulfate is the sulfate salt of Polymyxins B1 and B2, which are produced by the growth of Bacillus polymyxa (Prazmow... |
| Active Ingredient | Polymyxin b sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500,000 u base/vial |
| Market Status | Prescription |
| Company | X Gen Pharms; Xellia Pharms Aps; Fresenius Kabi Usa; Eurohlth Intl |
| 2 of 2 | |
|---|---|
| Drug Name | Polymyxin b sulfate |
| Drug Label | Polymyxin B for Injection, USP is one of a group of basic polypeptide antibiotics derived from B polymyxa (B aerosporous). Polymyxin B sulfate is the sulfate salt of Polymyxins B1 and B2, which are produced by the growth of Bacillus polymyxa (Prazmow... |
| Active Ingredient | Polymyxin b sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500,000 u base/vial |
| Market Status | Prescription |
| Company | X Gen Pharms; Xellia Pharms Aps; Fresenius Kabi Usa; Eurohlth Intl |
 ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
 Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38267
Submission : 2023-03-29
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35892
Submission : 2021-07-08
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 31587
Submission : 2017-03-15
Status : Active
Type : II

NDC Package Code : 59838-101
Start Marketing Date : 2020-02-24
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13199
Submission : 1998-04-30
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?