
Synopsis
Synopsis
0
EU WC
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
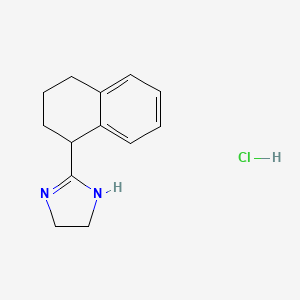

1. 2-(1,2,3,4-tetrahydro-1-naphthyl)-2-imidazoline
2. Berberil N
3. Caltheon
4. Collyrium Fresh
5. Diabenyl T
6. Eye-sine
7. Eye-zine
8. Murine Plus
9. Murine Sore Eyes
10. Ophtalmin
11. Optazine Fresh
12. Optigene
13. Rhinopront
14. Tetra-ide
15. Tetraclear
16. Tetrahydrozoline
17. Tetrahydrozoline Monohydrochloride
18. Tetrahydrozoline, (+-)-isomer
19. Tetrahydrozoline, (-)-isomer
20. Tetrilin
21. Tetryzoline
22. Tyzine
23. Vasopos
24. Visine
25. Vispring
26. Yxin
1. 522-48-5
2. Tetrahydrozoline Hcl
3. Tetryzoline Hydrochloride
4. Tyzine
5. Visine
6. Murine Plus
7. Vasopos
8. Tyzanol Hydrochloride
9. 2-(1,2,3,4-tetrahydronaphthalen-1-yl)-4,5-dihydro-1h-imidazole Hydrochloride
10. Tetrahydrozoline (hydrochloride)
11. Nsc-757339
12. 0yzt43hs7d
13. Mls000069739
14. 2-(1,2,3,4-tetrahydro-1-naphthyl)-2-imidazoline Hydrochloride
15. 2-(1,2,3,4-tetrahydro-1-naphthyl)-2-imidazoline Monohydrochloride
16. 1h-imidazole, 4,5-dihydro-2-(1,2,3,4-tetrahydro-1-naphthalenyl)-, Monohydrochloride
17. 2-tetralin-1-yl-4,5-dihydro-1h-imidazole Hydrochloride
18. 522-48-5 (hcl)
19. Smr000058219
20. 2-(1,2,3,4-tetrahydronaphthalen-1-yl)-4,5-dihydro-1h-imidazole;hydrochloride
21. 1h-imidazole, 4,5-dihydro-2-(1,2,3,4-tetrahydro-1-naphthalenyl)-, Hydrochloride (1:1)
22. Dsstox_cid_25316
23. Dsstox_rid_80797
24. Dsstox_gsid_45316
25. Rhinopront
26. Yxin
27. 2-(1,2,3,4-tetrahydronaphthalen-1-yl)-4,5-dihydro-1h-imidazole Hcl
28. Sr-01000000161
29. Einecs 208-329-3
30. Unii-0yzt43hs7d
31. Ai3-50165
32. Tetryzoline Hcl
33. Tetrahydrozoline Monohydrochloride
34. Tetrahydrozoline Hydrochloride [usp]
35. Mfcd00058029
36. Prestwick_1025
37. Tyzine (tn)
38. Opera_id_1724
39. Ncgc00016485-01
40. Cas-522-48-5
41. Schembl25702
42. Mls000079025
43. Mls001146954
44. Mls002222291
45. Mls002548902
46. Spectrum1500567
47. Chebi:9492
48. Chembl1200413
49. Dtxsid7045316
50. Hy-b0556a
51. Hms1570k17
52. Hms1921c21
53. Pharmakon1600-01500567
54. Bcp15857
55. Tox21_110450
56. Tox21_501137
57. Ccg-39268
58. Nsc757339
59. 2-imidazoline, 2-(1,2,3,4-tetrahydro-1-naphthyl)-, Hydrochloride
60. Akos015899540
61. Tox21_110450_1
62. Ac-1110
63. Lp01137
64. Nc00484
65. Nsc 757339
66. Tetrahydrozoline Hydrochloride, >=98%
67. Ncgc00016018-10
68. Ncgc00094403-01
69. Ncgc00094403-02
70. Ncgc00094403-03
71. Ncgc00094403-04
72. Ncgc00094403-05
73. Ncgc00261822-01
74. Tetryzoline Hydrochloride [mart.]
75. As-15973
76. Tetrahydrozoline Hydrochloride (jan/usp)
77. Tetryzoline Hydrochloride [who-dd]
78. Tetrahydrozoline Hydrochloride [mi]
79. Tetrahydrozoline Hydrochloride [jan]
80. Eu-0101137
81. Ft-0675067
82. S4043
83. Sw197098-3
84. T3148
85. Tetrahydrozoline Hydrochloride [vandf]
86. Vu0239726-5
87. D01023
88. T 4264
89. Tetrahydrozoline Hydrochloride [usp-rs]
90. Tetryzoline Hydrochloride [ep Monograph]
91. 522t485
92. A851840
93. Q-201816
94. Sr-01000000161-2
95. Sr-01000000161-8
96. Tetrahydrozoline Hydrochloride [orange Book]
97. Q27237368
98. Tetrahydrozoline Hydrochloride [usp Monograph]
99. Z1695906783
100. 1h-imidazole, 4,5-dihydro-2-(1,2,3,4-tetrahydro-1-naphthalenyl)-,monohydrochloride
101. 2-(1,2,3,4-tetrahydronaphthalen-1-yl)-4,5-dihydro-1h-imidazolehydrochloride
102. 4,5-dihydro-2-(1,2,3,4-tetrahydro-1-naphthalenyl)-1h-imidazole Hydrochloride
103. 4,5-dihydro-2-(1,2,3,4-tetrahydro-1-naphthalenyl)-1h-imidazole Monohydrochloride
104. Tetrahydrozoline Hydrochloride, European Pharmacopoeia (ep) Reference Standard
105. Tetrahydrozoline Hydrochloride, United States Pharmacopeia (usp) Reference Standard
106. Tetrahydrozoline Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 236.74 g/mol |
|---|---|
| Molecular Formula | C13H17ClN2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 236.1080262 g/mol |
| Monoisotopic Mass | 236.1080262 g/mol |
| Topological Polar Surface Area | 24.4 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 259 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Tyzine |
| Drug Label | Tyzine Nasal Solution contains tetrahydrozoline hydrochloride, 2-(1,2,3,4-Tetrahydro-1, naphthyl)-2-imidazoline monohydrochloride, as a nasal decongestant. The chemical structure is:Nasal Solution is available for topical nasal application as 0.1%... |
| Active Ingredient | Tetrahydrozoline hydrochloride |
| Dosage Form | Spray; Solution |
| Route | Nasal |
| Strength | 0.05%; 0.1% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Tyzine |
| Drug Label | Tyzine Nasal Solution contains tetrahydrozoline hydrochloride, 2-(1,2,3,4-Tetrahydro-1, naphthyl)-2-imidazoline monohydrochloride, as a nasal decongestant. The chemical structure is:Nasal Solution is available for topical nasal application as 0.1%... |
| Active Ingredient | Tetrahydrozoline hydrochloride |
| Dosage Form | Spray; Solution |
| Route | Nasal |
| Strength | 0.05%; 0.1% |
| Market Status | Prescription |
| Company | Fougera Pharms |
Ophthalmic Solutions
Sterile solutions that are intended for instillation into the eye. It does not include solutions for cleaning eyeglasses or CONTACT LENS SOLUTIONS. (See all compounds classified as Ophthalmic Solutions.)
Nasal Decongestants
Drugs designed to treat inflammation of the nasal passages, generally the result of an infection (more often than not the common cold) or an allergy related condition, e.g., hay fever. The inflammation involves swelling of the mucous membrane that lines the nasal passages and results in inordinate mucus production. The primary class of nasal decongestants are vasoconstrictor agents. (From PharmAssist, The Family Guide to Health and Medicine, 1993) (See all compounds classified as Nasal Decongestants.)
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.

Certificate Number : R0-CEP 2019-180 - Rev 00
Issue Date : 2021-09-28
Type : Chemical
Substance Number : 2101
Status : Valid

NDC Package Code : 51014-7162
Start Marketing Date : 2017-09-07
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Samoh Pharmaceutical Co., Ltd.
Registration Date : 2024-03-11
Registration Number : 20240311-211-J-1616
Manufacturer Name : PCAS Finland Oy
Manufacturer Address : Messukentankatu 8, 20210 Turku, Finland

| Available Reg Filing : ASMF |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5147
Submission : 1983-10-28
Status : Active
Type : II

Registration Number : 218MF10080
Registrant's Address : 50066 Reggello (Firenze) Italy
Initial Date of Registration : 2006-01-27
Latest Date of Registration : --

NDC Package Code : 12660-0048
Start Marketing Date : 1979-02-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15191
Submission : 2000-12-15
Status : Inactive
Type : II



 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
TETRAHYDROZOLINE HYDROCHLORIDE
Brand Name : TYZINE
Dosage Form : SOLUTION;NASAL
Dosage Strength : 0.1%
Approval Date : 1982-01-01
Application Number : 86576
RX/OTC/DISCN : RX
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
TETRAHYDROZOLINE HYDROCHLORIDE
Brand Name : TYZINE
Dosage Form : SOLUTION;NASAL
Dosage Strength : 0.05%
Approval Date : 1982-01-01
Application Number : 86576
RX/OTC/DISCN : RX
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
TETRAHYDROZOLINE HYDROCHLORIDE
Brand Name : TYZINE
Dosage Form : SPRAY;NASAL
Dosage Strength : 0.1%
Approval Date : 1982-01-01
Application Number : 86576
RX/OTC/DISCN : RX
RLD : No
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Dosage Form : Cream / Lotion / Ointment
Grade : Topical
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Injectable / Parenteral
Grade : Parenteral
Brand Name : Potassium Chloride
Application : Parenteral
Excipient Details : Potassium chloride is used as a diuretic-osmotic in injectable and parenteral solutions.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral, Topical
Brand Name : MONTANOX 80 PHA PREMIUM
Application : Emulsifying Agents
Pharmacopoeia Ref : USP-NF, EP, JP
Technical Specs : Non-Ionic Hydrophilic surfactant, Emulsifier (o/w emulsion), Solubilizer
Ingredient(s) : Polysorbate 80
Dosage Form : Emulsion
Grade : Topical
Application : Rheology Modifiers
Excipient Details : Thickener, Emulsifier, Stabilizer, Texturizing agent, pH Independent & Non Thixotropic polymer for Topical Range (Skin,Vaginal & Anal mucosa)
Pharmacopoeia Ref : In house having US DMF Type IV...
Technical Specs : Ready to use liquid polymer for topical applications (Gel / Cream / Lotion/ Foam based formulation, ...
Ingredient(s) : Hydroxyethyl Acrylate
Dosage Form : Capsule
Grade : Oral
Application : Solubilizers
Excipient Details : Polysorbate 80 in dry powder form, a solubilizing agent acts as a surfactant and increases the solubility of various oral dosage forms.
Pharmacopoeia Ref : USP-NF, EP, JP & having US DMF
Technical Specs : Solubilizer in powder form, used in directly compressible dosage forms, Wet granulation, added durin...
Ingredient(s) : Magnesium aluminium silicate Excipient
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral, Topical & Parenteral
Application : Solubilizers
Excipient Details : Polysorbate 80 acts as solubilizer, emulsifier and wetting agent.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Reply
08 Oct 2024
Reply
23 Sep 2024
Reply
02 Mar 2024
Reply
25 Oct 2023
Reply
17 Jun 2023
Reply
17 Jan 2023
Reply
15 Jul 2022
Reply
16 Oct 2020
Reply
06 Oct 2020
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?