09 Jan 2025
// PRESS RELEASE
09 Jan 2025
// PRESS RELEASE
25 Nov 2024
// PRESS RELEASE
Latest Content by PharmaCompass

 KEY PRODUCTS
KEY PRODUCTS

![]() Reset all filters
Reset all filters
01 1PURIFIED YOLK LECITHIN DRUG EXCIPIENT

![]() Reset all filters
Reset all filters
01 1KEWPIE CORP

![]() Reset all filters
Reset all filters
01 1Japan

![]() Reset all filters
Reset all filters
01 1Active

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15449
Submission : 2001-05-22
Status : Active
Type : IV
Excipients Web Link
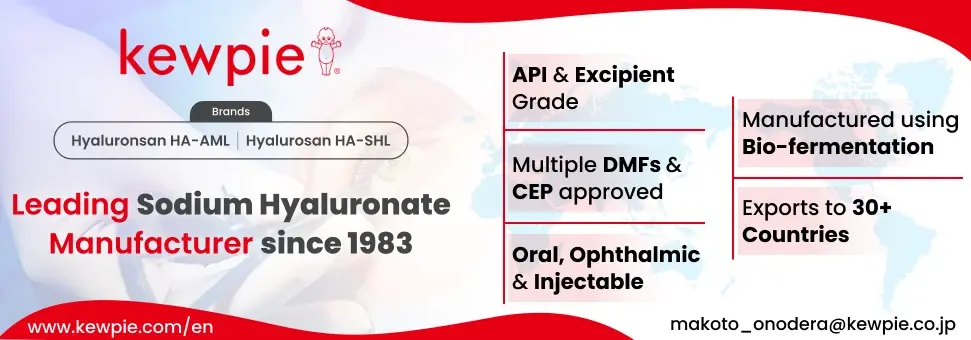
Kewpie Corporation is a supplier offers 5 products (APIs, Excipients or Intermediates).
Find a price of Sodium Hyaluronate bulk with DMF, CEP, JDMF offered by Kewpie Corporation
Find a price of Sodium Hyaluronate bulk with DMF, JDMF offered by Kewpie Corporation
Find a price of Hyaluronic Acid bulk with JDMF offered by Kewpie Corporation
Find a price of Sodium Hyaluronate bulk with JDMF offered by Kewpie Corporation
Find a price of Sodium Hyaluronate bulk offered by Kewpie Corporation