
Synopsis
Synopsis
0
JDMF
0
VMF
0
Australia

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
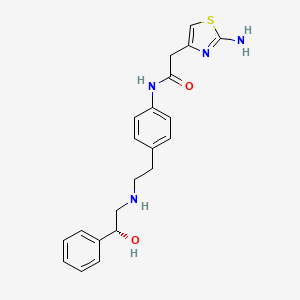

1. 2-(2-aminothiazol-4-yl)-4'-(2-((2-hydroxy-2-phenylethyl)amino)ethyl)acetanilide
2. Betanis
3. Betmiga
4. Ym 178
5. Ym-178
1. 223673-61-8
2. Myrbetriq
3. Betanis
4. Betmiga
5. Ym178
6. Mirabegron (ym178)
7. Ym-178
8. Ym 178
9. 2-(2-amino-1,3-thiazol-4-yl)-n-[4-(2-{[(2r)-2-hydroxy-2-phenylethyl]amino}ethyl)phenyl]acetamide
10. Mvr3jl3b2v
11. Chebi:65349
12. 2-amino-n-[4-[2-[[(2r)-2-hydroxy-2-phenylethyl]amino]ethyl]phenyl]-4-thiazoleacetamide
13. (r)-2-(2-aminothiazol-4-yl)-n-(4-(2-((2-hydroxy-2-phenylethyl)amino)ethyl)phenyl)acetamide
14. 4-thiazoleacetamide, 2-amino-n-(4-(2-(((2r)-2-hydroxy-2-phenylethyl)amino)ethyl)phenyl)-
15. Myrbetriq (tn)
16. 2-(2-amino-1,3-thiazol-4-yl)-n-[4-[2-[[(2r)-2-hydroxy-2-phenylethyl]amino]ethyl]phenyl]acetamide
17. 2-(2-azanyl-1,3-thiazol-4-yl)-n-[4-[2-[[(2r)-2-oxidanyl-2-phenyl-ethyl]amino]ethyl]phenyl]ethanamide
18. Mirabegron [usan:inn]
19. Unii-mvr3jl3b2v
20. 2-(2-amino-1,3-thiazol-4-yl)-n-(4-(2-(((2r)-2-hydroxy-2-phenylethyl)amino)ethyl)phenyl)acetamide
21. Mirabegron [mi]
22. Mirabegron [inn]
23. Mirabegron [jan]
24. Mirabegron (usan/jan)
25. Mirabegron [usan]
26. Mirabegron [vandf]
27. Mirabegron [mart.]
28. Mirabegron [who-dd]
29. N-(4-(2-(2-hydroxy-2-phenylethylamino)ethyl)phenyl)-2-(2-aminothiazol-4-yl)acetamide
30. Schembl904788
31. Gtpl7445
32. Chembl2095212
33. Mirabegron [orange Book]
34. Amy1800
35. Dtxsid101021648
36. Hms3714i09
37. Hms3885m16
38. 2-(2-amino-1,3-thiazol-4-yl)-n-(4-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl}phenyl)acetamide
39. Ex-a1050
40. Zinc1996784
41. Mfcd11100356
42. S4009
43. Akos016340341
44. Ccg-268611
45. Cs-0915
46. Db08893
47. Ks-1398
48. 2-(2-aminothiazol-4-yl)-4'-(2-((2-hydroxy-2-phenylethyl)amino)ethyl)acetanilide
49. Ncgc00386239-01
50. Hy-14773
51. Sw220301-1
52. D09535
53. Ab01565808_02
54. A816162
55. Ar-270/43507997
56. Q3702534
57. (r)-2-(2-aminothiazol-4-yl)-4'-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetanilide
58. (r)-2-(2-aminothiazol-4-yl)-4-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl}acetanilide
59. (r)-2-(2-aminothiazol-4-yl)-n-(4-(2-(2-hydroxy-2-phenylethylamino)ethyl)phenyl)acetamide
60. 2-(2-aminothiazol-4-yl)-n-(4-(2-(((2r)-2-hydroxy-2-phenylethyl)amino)ethyl)phenyl)acetamide
61. H6u
62. Ym 178;2-(2-aminothiazol-4-yl)-n-[4-[2-[[(2r)-2-hydroxy-2-phenyl-ethyl]amino]ethyl]phenyl]acetamide
| Molecular Weight | 396.5 g/mol |
|---|---|
| Molecular Formula | C21H24N4O2S |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 396.16199719 g/mol |
| Monoisotopic Mass | 396.16199719 g/mol |
| Topological Polar Surface Area | 129 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 467 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Myrbetriq |
| PubMed Health | Mirabegron (Oral route) |
| Drug Label | Mirabegron is a beta-3 adrenergic agonist. The chemical name is 2-(2-aminothiazol-4-yl)-N-[4-(2-{[(2R)-2-hydroxy-2-phenylethyl]amino}ethyl)phenyl]acetamide having an empirical formula of C21H24N4O2S and a molecular weight of 396.51. The structural fo... |
| Active Ingredient | Mirabegron |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 25mg; 50mg |
| Market Status | Prescription |
| Company | Apgdi |
| 2 of 2 | |
|---|---|
| Drug Name | Myrbetriq |
| PubMed Health | Mirabegron (Oral route) |
| Drug Label | Mirabegron is a beta-3 adrenergic agonist. The chemical name is 2-(2-aminothiazol-4-yl)-N-[4-(2-{[(2R)-2-hydroxy-2-phenylethyl]amino}ethyl)phenyl]acetamide having an empirical formula of C21H24N4O2S and a molecular weight of 396.51. The structural fo... |
| Active Ingredient | Mirabegron |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 25mg; 50mg |
| Market Status | Prescription |
| Company | Apgdi |
Mirabegron is indicated for the treatment of overactive bladder (OAB) - with symptoms of urge urinary incontinence, urgency, and urinary frequency - either alone or in combination with [solifenacin]. It is also indicated for the treatment of neurogenic detrusor overactivity (NDO) in pediatric patients 3 years of age and older and weighing 35kg or more.
Symptomatic treatment of urgency.
Increased micturition frequency and / or urgency incontinence as may occur in adult patients with overactive-bladder syndrome.
Treatment of idiopathic overactive bladder
Treatment of neurogenic detrusor overactivity
Mirabegron exerts its pharmacologic effects by forcing bladder smooth muscle to relax, thereby expanding its capacity and relieving urgency. Mirabegron does not appear to adversely affect the mean maximum flow rate or mean detrusor pressure at maximum flow rate in patients with lower urinary tract symptoms and bladder outlet obstruction (BOO), but should be used with in patients with BOO due to reports of significant urinary retention. Furthermore, mirabegron increases both blood pressure and heart rate in a dose-dependent manner and should therefore be used with caution in patients with severely uncontrolled hypertension or others for whom these increases may prove dangerous.
Adrenergic beta-3 Receptor Agonists
Compounds that bind to and activate ADRENERGIC BETA-3 RECEPTORS. (See all compounds classified as Adrenergic beta-3 Receptor Agonists.)
Urological Agents
Drugs used in the treatment of urological conditions and diseases such as URINARY INCONTINENCE and URINARY TRACT INFECTIONS. (See all compounds classified as Urological Agents.)
G04BD12
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BD - Drugs for urinary frequency and incontinence
G04BD12 - Mirabegron
Absorption
The absolute bioavailability of orally administered mirabegron ranges from 29% at a dose of 25 mg to 35% at a dose of 50 mg. The Tmax for the extended-release tablet and suspension formulations are approximately 3.5 hours, while the Tmax for the granule formulation is 4-5 hours. Both Cmax and AUC increase more than dose proportionally - an increase in dose from 50mg to 100mg results in a 2.9- and 2.6-fold increase in Cmax and AUC, respectively, whereas an increase from 50mg to 200mg results in a 8.4- and 6.5-fold increase in Cmax and AUC, respectively. Steady-state concentrations of mirabegron are achieved after approximately 7 days of once-daily administration.
Route of Elimination
Of a 160mg radiolabeled dose administered to healthy volunteers, approximately 55% of the radioactivity was recovered in the urine and 34% in the feces. Approximately 25% of unchanged mirabegron was recovered in the urine while 0% was recovered in the feces. Renal elimination is achieved primarily via active tubular secretion with some contribution by glomerular filtration.
Volume of Distribution
Following intravenous administration, mirabegron has an apparent steady-state volume of distribution (Vd) of 1670 L indicating extensive distribution.
Clearance
Total plasma clearance following intravenous administration is approximately 57 L/h, with renal clearance accounting for roughly 25% at approximately 13 L/h.
Mirabegron is extensively metabolized via a number of mechanisms, although unchanged parent drug is still the major circulating component following oral administration. Presumed metabolic pathways and their resultant metabolites include amide hydrolysis (M5, M16, M17), glucuronidation (mirabegron O-glucuronide, N-glucuronide, N-carbamoylglucuronide, M12), and secondary amine oxidation or dealkylation (M8, M9, M15), amongst others. The enzymes responsible for the oxidative metabolism of mirabegron are thought to be CYP3A4 and CYP2D6, while the UDP-glucuronosyltransferases responsible for conjugation reactions have been identified as UGT2B7, UGT1A3, and UGT1A8. Other enzymes that may be involved in the metabolism of mirabegron include butylcholinesterase and possibly alcohol dehydrogenase.
The mean terminal elimination half-life of mirabegron in adults being treated for overactive bladder is approximately 50 hours. In pediatric patients receiving the granule formulation for the treatment of neurogenic detrusor overactivity, the mean terminal elimination half-life is approximately 26-31 hours.
Mirabegron is a potent and selective agonist of beta-3 adrenergic receptors. The activation of beta-3 receptors relaxes detrusor smooth muscle during the storage phase of the urinary bladder fill-void cycle, which increases the bladder's storage capacity thereby alleviating feelings of urgency and frequency.
 Synnat Pharma is one of the leading active pharmaceutical ingredients and intermediates manufacturers.
Synnat Pharma is one of the leading active pharmaceutical ingredients and intermediates manufacturers.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38770
Submission : 2023-08-31
Status : Active
Type : II

Certificate Number : CEP 2023-314 - Rev 00
Issue Date : 2024-12-11
Type : Chemical
Substance Number : 3132
Status : Valid

NDC Package Code : 58032-2040
Start Marketing Date : 2023-03-30
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
 Minakem delivers API, HPAPI, steroids & CDMO services for generics with FDA/GMP certification, regulatory know-how & proven success.
Minakem delivers API, HPAPI, steroids & CDMO services for generics with FDA/GMP certification, regulatory know-how & proven success.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.

GDUFA
DMF Review : Reviewed
Rev. Date : 2014-09-29
Pay. Date : 2014-07-14
DMF Number : 28392
Submission : 2014-06-30
Status : Active
Type : II

Date of Issue : 2022-06-17
Valid Till : 2025-07-07
Written Confirmation Number : WC-0067
Address of the Firm :

NDC Package Code : 55111-961
Start Marketing Date : 2014-06-30
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Chong Kun Dang Co., Ltd.
Registration Date : 2020-08-27
Registration Number : No. 574-10-ND(2)
Manufacturer Name : Dr. Reddy's Laboratories Limited
Manufacturer Address : Chemical Technical Operations-Unit VI, APIIC Industrial Estate, Pydibhimavaram, Ranasthalam Mandal, Srikakulam District, Andhra Pradesh, India

| Available Reg Filing : BR, ASMF, CA, EG |
 Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.

GDUFA
DMF Review : Reviewed
Rev. Date : 2020-12-03
Pay. Date : 2018-07-09
DMF Number : 32929
Submission : 2018-09-14
Status : Active
Type : II
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36973
Submission : 2022-04-27
Status : Active
Type : II

NDC Package Code : 42765-047
Start Marketing Date : 2022-04-14
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38770
Submission : 2023-08-31
Status : Active
Type : II
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
GDUFA
DMF Review : Complete
Rev. Date : 2015-05-19
Pay. Date : 2015-03-04
DMF Number : 29064
Submission : 2015-02-27
Status : Active
Type : II
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
GDUFA
DMF Review : Complete
Rev. Date : 2014-09-29
Pay. Date : 2014-07-14
DMF Number : 28392
Submission : 2014-06-30
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2020-12-03
Pay. Date : 2018-07-09
DMF Number : 32929
Submission : 2018-09-14
Status : Active
Type : II
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36973
Submission : 2022-04-27
Status : Active
Type : II
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33821
Submission : 2019-04-26
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40421
Submission : 2024-10-24
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2016-02-26
Pay. Date : 2016-01-14
DMF Number : 29925
Submission : 2015-12-31
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2016-02-05
Pay. Date : 2015-09-30
DMF Number : 29773
Submission : 2015-09-25
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2016-04-27
Pay. Date : 2015-09-28
DMF Number : 29734
Submission : 2015-09-28
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Mirabegron Amorphous IH/Mirabegron IH
Date of Issue : 2022-06-17
Valid Till : 2025-07-07
Written Confirmation Number : WC-0067
Address of the Firm : Chemical Technical Operations Unit-VI, APIIC Industrial Estate, Pydibhimavaram V...
Date of Issue : 2022-05-06
Valid Till : 2025-07-02
Written Confirmation Number : WC-0100N1
Address of the Firm : Plot Number 2,3,4 & 5, Phase-IV, Bommasandra-Jigani Link Road, Bommasandra Post,...

Date of Issue : 2022-06-24
Valid Till : 2025-07-02
Written Confirmation Number : WC-0097
Address of the Firm : Block No. 251/P, 252/P, 253 to 255, 256/P,258/P, 276/P, 277/P, 278/P, 279 To 282...

Date of Issue : 2023-12-28
Valid Till : 2026-12-28
Written Confirmation Number : WC-0310
Address of the Firm : Plot No. Z-103/l, Dahej SEZ, Phase ll, Dahej, Dist-Bharuch, Gujarat

Date of Issue : 2019-08-09
Valid Till : 2025-08-08
Written Confirmation Number : WC-0066
Address of the Firm : Plot No. 1, Hetero SEZ Infrastructure Ltd., Narasapuram, Visakhapatnam-531 081, ...

Date of Issue : 2022-06-07
Valid Till : 2025-06-16
Written Confirmation Number : WC-0049
Address of the Firm : Block 21, Dabhasa, Padra Taluka, Vadodara-391 440

Date of Issue : 2022-07-27
Valid Till : 2025-07-21
Written Confirmation Number : WC-0087-(Annexure-1)
Address of the Firm : Plot No.1 to 5, 31 to 35 & 48 to 51, 26 & K/201, Village Lakhmapur, Taluka Dindo...

Date of Issue : 2020-02-10
Valid Till : 2022-08-15
Written Confirmation Number : WC-0225A6
Address of the Firm : Plot No 43-45 Bommasandra Industrial area, 4th Phase, Anekal taluk,Bengaluru-560...

Date of Issue : 2022-01-04
Valid Till : 2022-07-14
Written Confirmation Number : WC-0021A4
Address of the Firm : Sy. No.317&323, Rudraram Village, Patancheru Mandal, Sangaredy District-502329 T...

Date of Issue : 2019-07-15
Valid Till : 2022-07-14
Written Confirmation Number : WC-0021
Address of the Firm : Sy. No.317&323, Rudraram Village, Patancheru Mandal, Sangaredy District-502329 T...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Synnat Pharma is one of the leading active pharmaceutical ingredients and intermediates manufacturers.
Synnat Pharma is one of the leading active pharmaceutical ingredients and intermediates manufacturers.
About the Company : Synnat Pharma is a fast-growing pharmaceutical company dedicated to the identification, development, production and distribution of potent phytochemicals and botanical extracts. AP...
About the Company : Established in 1984, Neuland Laboratories Limited is a publicly listed company headquartered in Hyderabad, India. The company provides solutions across the entire spectrum of the p...
 Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
About the Company : Aarti Pharmalab, earlier the pharma division of Aarti Industries, is a leading Indian manufacturer of APIs. It has dedicated facilities to manufacture HPAPIs, corticosteroids, cyto...
 Minakem delivers API, HPAPI, steroids & CDMO services for generics with FDA/GMP certification, regulatory know-how & proven success.
Minakem delivers API, HPAPI, steroids & CDMO services for generics with FDA/GMP certification, regulatory know-how & proven success.
About the Company : MINAKEM is a cGMP custom manufacturer of small molecule API, HPAPI and steroids. All development and manufacturing activities are supported by highly skilled R&D teams, supported b...
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About the Company : Founded in 1984, DRL is well-known for its generic APIs and its track record in drug product development. It is one of the earliest pharma API manufacturers with a diverse portfoli...
 Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
About the Company : Zeal MediPharma is a globally recognized Star One Export House, serving customers in over 50 countries for more than two decades. We specialize in sourcing and exporting high-quali...
About the Company : Minakem Montreal is developing and manufacturing small molecules APIs and advanced intermediates, including corticosteroids. Following efficient processes and methodologies, our em...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product gro...
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
About the Company : Established in 1994, Rochem is a distributor of pharmaceutical, food, nutritional and animal health ingredients to some of the largest companies in the world. It sources high-quali...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Regulatory Info :
Registration Country : Greece
Brand Name :
Dosage Form : Prolonged Release Tablet
Dosage Strength : 25MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Greece
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Regulatory Info :
Registration Country : Greece
Brand Name :
Dosage Form : Prolonged Release Tablet
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Greece
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 50MG
Packaging :
Approval Date : 2024-02-12
Application Number : 215948
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Betmiga
Dosage Form : PROLONGED-RELEASE TABLET
Dosage Strength : 25 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Betmiga
Dosage Form : Depottablett
Dosage Strength : 25 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Betmiga
Dosage Form : Depottablett
Dosage Strength : 50 mg
Packaging : Bottle
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Betmiga
Dosage Form : Depot tablet
Dosage Strength : 25 mg
Packaging : Blisterpakning 90item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Betmiga 25 mg
Dosage Form : TAB
Dosage Strength : 25mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : USA
Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number : 209413
Regulatory Info :
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : ER Tablet
Dosage Strength : 25MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 25MG
Approval Date : 2024-02-12
Application Number : 215948
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 50MG
Approval Date : 2024-02-12
Application Number : 215948
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : MYRBETRIQ GRANULES
Dosage Form : FOR SUSPENSION, EXTENDED RELEASE;ORAL
Dosage Strength : 8MG/ML
Approval Date : 2021-03-25
Application Number : 213801
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 50MG
Approval Date : 2025-01-02
Application Number : 209434
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 25MG
Approval Date : 2022-09-28
Application Number : 209485
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 50MG
Approval Date : 2022-09-28
Application Number : 209485
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 25MG
Approval Date : 2019-12-27
Application Number : 209446
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 25MG
Approval Date : 2022-09-29
Application Number : 209488
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 50MG
Approval Date : 2022-09-29
Application Number : 209488
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

Brand Name : MIRABEGRON
Dosage Form : TABLET, EXTENDED RELEASE
Dosage Strength : 50MG
Approval Date :
Application Number : 217989
RX/OTC/DISCN :
RLD :
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Betmiga
Dosage Form : PROLONGED-RELEASE TABLET
Dosage Strength : 25 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Betmiga
Dosage Form : Depottablett
Dosage Strength : 25 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Betmiga
Dosage Form : Depottablett
Dosage Strength : 25 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Betmiga
Dosage Form : Depottablett
Dosage Strength : 50 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Betmiga
Dosage Form : Depottablett
Dosage Strength : 50 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Betmiga
Dosage Form : Depot tablet
Dosage Strength : 50 mg
Packaging : Blisterpakning 30item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Betmiga
Dosage Form : Depot tablet
Dosage Strength : 25 mg
Packaging : Blisterpakning 90item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Betmiga
Dosage Form : Ret Tablet
Dosage Strength : 25mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Betmiga
Dosage Form : Ret Tablet
Dosage Strength : 25mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Betmiga
Dosage Form : Ret Tablet
Dosage Strength : 50mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Canada
Brand Name : MYRBETRIQ
Dosage Form : TABLET (EXTENDED-RELEASE)
Dosage Strength : 25MG
Packaging : 30/90
Approval Date :
Application Number : 2402874
Regulatory Info :
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Canada
Brand Name : MYRBETRIQ
Dosage Form : TABLET (EXTENDED-RELEASE)
Dosage Strength : 50MG
Packaging : 30/90
Approval Date :
Application Number : 2402882
Regulatory Info :
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Betmiga 25 mg
Dosage Form : TAB
Dosage Strength : 25mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Betmiga 50 mg
Dosage Form : TAB
Dosage Strength : 50mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Regulatory Info :
Registration Country : Greece
Brand Name :
Dosage Form : Prolonged Release Tabl...
Dosage Strength : 25MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Greece
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Packaging :
Regulatory Info :
Dosage : Prolonged Release Tabl...
Dosage Strength : 25MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Greece
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Regulatory Info :
Registration Country : Greece
Brand Name :
Dosage Form : Prolonged Release Tabl...
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Greece
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Packaging :
Regulatory Info :
Dosage : Prolonged Release Tabl...
Dosage Strength : 50MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Greece
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 25MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : Tablet
Dosage Strength : 25MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : Tablet
Dosage Strength : 50MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Portugal
Brand Name :
Dosage Form : Prolonged Release Tabl...
Dosage Strength : 25MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Portugal

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Prolonged Release Tabl...
Dosage Strength : 25MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Portugal

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Portugal
Brand Name :
Dosage Form : Prolonged Release Tabl...
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Portugal

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Prolonged Release Tabl...
Dosage Strength : 50MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Portugal

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : ER Tablet
Dosage Strength : 25MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : ER Tablet
Dosage Strength : 25MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : ER Tablet
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : ER Tablet
Dosage Strength : 50MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : South Korea
Brand Name : Bemiga SR
Dosage Form : Tablet
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : South Korea

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 50MG
Brand Name : Bemiga SR
Approval Date :
Application Number :
Registration Country : South Korea

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS


08 Jan 2025
// FDA
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=209434

08 Oct 2024
// PRESS RELEASE
https://en.hengkangpharm.cn/index.php/news/cphi-milan-2024-zhejiang-hengkang-pharm/

04 Sep 2024
// EXPRESSPHARMA
https://www.expresspharma.in/lupin-launches-mirabegron-extended-release-tablets-in-the-us/

22 Apr 2024
// BUSINESSWIRE

20 Apr 2024
// Phil Taylor PHARMAPHORUM
https://pharmaphorum.com/news/astellas-generic-myrbetriq-defence-knocked-back-again

12 Feb 2024
// FDA
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=215948
Global Sales Information

Company :
Mirabegron
Drug Cost (USD) : 2,252,501,068
Year : 2022
Prescribers : 716234
Prescriptions : 3458632

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Mirabegron
Drug Cost (USD) : 1,989,099,248
Year : 2021
Prescribers : 665158
Prescriptions : 3233926

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Mirabegron
Drug Cost (USD) : 1,749,232,347
Year : 2020
Prescribers : 600136
Prescriptions : 3072674

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Mirabegron
Drug Cost (USD) : 1,445,078,415
Year : 2019
Prescribers : 564887
Prescriptions : 2773085

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Mirabegron
Drug Cost (USD) : 1,063,585,890
Year : 2018
Prescribers : 464893
Prescriptions : 2261032

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Mirabegron
Drug Cost (USD) : 787,303,712
Year : 2017
Prescribers : 395225
Prescriptions : 1881245

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Mirabegron
Drug Cost (USD) : 518,607,486
Year : 2016
Prescribers : 296979
Prescriptions : 1399013

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Mirabegron
Drug Cost (USD) : 319,584,006
Year : 2015
Prescribers : 213691
Prescriptions : 944637

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
MIRABEGRON
Drug Cost (USD) : 175,833,979
Year : 2014
Prescribers : 147562
Prescriptions : 585502

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Depot tablet
Dosage Strength : 50 mg
Price Per Pack (Euro) : 26.38
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Depot tablet
Dosage Strength : 50 mg
Price Per Pack (Euro) : 76.57
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Depot tablet
Dosage Strength : 25 mg
Price Per Pack (Euro) : 26.38
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Depot tablet
Dosage Strength : 25 mg
Price Per Pack (Euro) : 76.57
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Depottablett
Dosage Strength : 25 mg
Price Per Pack (Euro) : 40.909
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Depottablett
Dosage Strength : 25 mg
Price Per Pack (Euro) : 113.25
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Depottablett
Dosage Strength : 50 mg
Price Per Pack (Euro) : 40.909
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Depottablett
Dosage Strength : 50 mg
Price Per Pack (Euro) : 113.25
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Depottablett
Dosage Strength : 50 mg
Price Per Pack (Euro) : 113.25
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Ret Tablet
Dosage Strength : 25mg
Price Per Pack (Euro) : 36.74
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Main Therapeutic Indication : Nephrology
Currency : USD
2020 Revenue in Millions : 1,492
2019 Revenue in Millions : 1,451
Growth (%) : 3

Main Therapeutic Indication : Nephrology
Currency : USD
2021 Revenue in Millions : 1,420
2020 Revenue in Millions : 1,481
Growth (%) : 3

Main Therapeutic Indication : Nephrology
Currency : USD
2022 Revenue in Millions : 1,437
2021 Revenue in Millions : 1,455
Growth (%) : -1

Main Therapeutic Indication : Nephrology
Currency : USD
2023 Revenue in Millions : 1,284
2022 Revenue in Millions : 1,437
Growth (%) : 1

Main Therapeutic Indication : Renal Disorders
Currency : USD
2018 Revenue in Millions : 1,287
2017 Revenue in Millions : 1,003
Growth (%) : 28%

Main Therapeutic Indication : Renal Disorders
Currency : USD
2016 Revenue in Millions : 839
2015 Revenue in Millions : 679
Growth (%) : 24

Main Therapeutic Indication : Nephrology
Currency : USD
2019 Revenue in Millions : 1,472
2018 Revenue in Millions : 1,325
Growth (%) : 11

Main Therapeutic Indication : Renal Disorders
Currency : USD
2017 Revenue in Millions : 1,070
2016 Revenue in Millions : 895
Growth (%) : 20

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
13 Sep 2024
Reply
10 Sep 2024
Reply
15 Jan 2024
Reply
14 Nov 2023
Reply
06 Jun 2023
Reply
05 May 2023
Reply
11 Feb 2023
Reply
06 Jul 2022
Reply
15 Apr 2021
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

CAS Number : 223673-61-8
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : M0056.00

(R)-2-(2-aminothiazol-4-yl)-N-(4-(2-(2-aminot...
CAS Number : 1684452-84-3
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No :

Mirabegran deshydroxy impurity
CAS Number : 1581284-82-3
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No :

CAS Number : 1684452-83-2
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No :

Mirabegron CRS
CAS Number : 223673-61-8
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No :

Mirabegron impurity B CRS
CAS Number : NA
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No :

Mirabegron impurity C CRS
CAS Number : 1581284-82-3
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No :

Mirabegron impurity F CRS
CAS Number : 223673-34-5
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No :

Mirabegron impurity G CRS
CAS Number : 521284-19-5
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No :

Mirabegron racemate CRS
CAS Number : NA
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
41
PharmaCompass offers a list of Mirabegron API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mirabegron manufacturer or Mirabegron supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mirabegron manufacturer or Mirabegron supplier.
PharmaCompass also assists you with knowing the Mirabegron API Price utilized in the formulation of products. Mirabegron API Price is not always fixed or binding as the Mirabegron Price is obtained through a variety of data sources. The Mirabegron Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Mirabegron manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Mirabegron, including repackagers and relabelers. The FDA regulates Mirabegron manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Mirabegron API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Mirabegron manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Mirabegron supplier is an individual or a company that provides Mirabegron active pharmaceutical ingredient (API) or Mirabegron finished formulations upon request. The Mirabegron suppliers may include Mirabegron API manufacturers, exporters, distributors and traders.
click here to find a list of Mirabegron suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Mirabegron DMF (Drug Master File) is a document detailing the whole manufacturing process of Mirabegron active pharmaceutical ingredient (API) in detail. Different forms of Mirabegron DMFs exist exist since differing nations have different regulations, such as Mirabegron USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Mirabegron DMF submitted to regulatory agencies in the US is known as a USDMF. Mirabegron USDMF includes data on Mirabegron's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Mirabegron USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Mirabegron suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Mirabegron Drug Master File in Korea (Mirabegron KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Mirabegron. The MFDS reviews the Mirabegron KDMF as part of the drug registration process and uses the information provided in the Mirabegron KDMF to evaluate the safety and efficacy of the drug.
After submitting a Mirabegron KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Mirabegron API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Mirabegron suppliers with KDMF on PharmaCompass.
A Mirabegron CEP of the European Pharmacopoeia monograph is often referred to as a Mirabegron Certificate of Suitability (COS). The purpose of a Mirabegron CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Mirabegron EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Mirabegron to their clients by showing that a Mirabegron CEP has been issued for it. The manufacturer submits a Mirabegron CEP (COS) as part of the market authorization procedure, and it takes on the role of a Mirabegron CEP holder for the record. Additionally, the data presented in the Mirabegron CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Mirabegron DMF.
A Mirabegron CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Mirabegron CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Mirabegron suppliers with CEP (COS) on PharmaCompass.
A Mirabegron written confirmation (Mirabegron WC) is an official document issued by a regulatory agency to a Mirabegron manufacturer, verifying that the manufacturing facility of a Mirabegron active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Mirabegron APIs or Mirabegron finished pharmaceutical products to another nation, regulatory agencies frequently require a Mirabegron WC (written confirmation) as part of the regulatory process.
click here to find a list of Mirabegron suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Mirabegron as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Mirabegron API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Mirabegron as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Mirabegron and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Mirabegron NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Mirabegron suppliers with NDC on PharmaCompass.
Mirabegron Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Mirabegron GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Mirabegron GMP manufacturer or Mirabegron GMP API supplier for your needs.
A Mirabegron CoA (Certificate of Analysis) is a formal document that attests to Mirabegron's compliance with Mirabegron specifications and serves as a tool for batch-level quality control.
Mirabegron CoA mostly includes findings from lab analyses of a specific batch. For each Mirabegron CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Mirabegron may be tested according to a variety of international standards, such as European Pharmacopoeia (Mirabegron EP), Mirabegron JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Mirabegron USP).
