
Synopsis
Synopsis
0
KDMF
0
VMF
0
FDA Orange Book

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
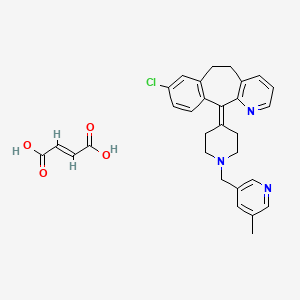

1. 182349-12-8
2. Rupatadin Fumarate
3. Rupafin
4. Ur-12592 Fumarate
5. Rupatadine (fumarate)
6. Alergoliber
7. Rinialer
8. Xj6ot32m93
9. 182349-12-8 (fumarate)
10. Rupax
11. 8-chloro-11-(1-((5-methylpyridin-3-yl)methyl)piperidin-4-ylidene)-6,11-dihydro-5h-benzo[5,6]cyclohepta[1,2-b]pyridine Fumarate
12. Rupatadine Fumarate (jan)
13. 1217234-48-4
14. Rupatadine Fumarate [jan]
15. (2e)-but-2-enedioic Acid; 13-chloro-2-{1-[(5-methylpyridin-3-yl)methyl]piperidin-4-ylidene}-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(15),3,5,7,11,13-hexaene
16. 5h-benzo[5,6]cyclohepta[1,2-b]pyridine, 8-chloro-6,11-dihydro-11-[1-[(5-methyl-3-pyridinyl)methyl]-4-piperidinylidene]-, (2e)-2-butenedioate (1:1)
17. Mfcd00926499
18. Unii-xj6ot32m93
19. Rupatall
20. Tamalis
21. Wystamm
22. Ralif
23. Rinialer (tn)
24. 5h-benzo(5,6)cyclohepta(1,2-b)pyridine, 8-chloro-6,11-dihydro-11-(1-((5-methyl-3-pyridinyl)methyl)-4-piperidinylidene)-, (2e)-2-butenedioate (1:1)
25. Rupafin (tn)
26. Rupatadine Fumarate- Bio-x
27. 5h-benzo(5,6)cyclohepta(1,2-b)pyridine, 6,11-dihydro-8-chloro-11-(1-((5-methyl-3-pyridinyl)methyl)-4-piperidinylidene)-, (e)-2-butenedioate (1:1)
28. 8-chloro-6,11-dihydro-11-[1-[(5-methyl-3-pyridyl)methyl]-4-piperidylidene]-5h-benzo[5,6]cyclohepta[1,2-b]pyridine Fumarate
29. Mls006010150
30. Schembl180121
31. Schembl180512
32. Rupatadine Fumarate [mi]
33. Hms3885k05
34. Bcp05230
35. Hy-13511a
36. Rupatadine Fumarate [who-dd]
37. S3052
38. Akos005145898
39. Ac-9016
40. Ccg-269922
41. Cs-3482
42. F76r825
43. Ks-1229
44. Br164385
45. Rupatadine Fumarate [ep Monograph]
46. Smr004701267
47. Sw219889-1
48. C73520
49. D08497
50. Q-201688
51. Q27293863
52. (e)-but-2-enedioic Acid;13-chloro-2-[1-[(5-methylpyridin-3-yl)methyl]piperidin-4-ylidene]-4-azatricyclo[9.4.0.03,8]pentadeca-1(11),3(8),4,6,12,14-hexaene
| Molecular Weight | 532.0 g/mol |
|---|---|
| Molecular Formula | C30H30ClN3O4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 531.1924841 g/mol |
| Monoisotopic Mass | 531.1924841 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 728 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Treatment of allergic rhinitis, Treatment of chronic idiopathic urticaria
 IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.

Certificate Number : CEP 2021-095 - Rev 00
Issue Date : 2024-07-10
Type : Chemical
Substance Number : 2888
Status : Valid
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.

Certificate Number : CEP 2023-016 - Rev 00
Issue Date : 2024-07-09
Type : Chemical
Substance Number : 2888
Status : Valid
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38451
Submission : 2023-05-31
Status : Active
Type : II

Certificate Number : R0-CEP 2018-180 - Rev 02
Issue Date : 2023-02-02
Type : Chemical
Substance Number : 2888
Status : Valid

Date of Issue : 2022-06-15
Valid Till : 2025-07-14
Written Confirmation Number : WC-0183nA2
Address of the Firm :

NDC Package Code : 66577-035
Start Marketing Date : 2018-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36047
Submission : 2021-08-09
Status : Active
Type : II

Certificate Number : R1-CEP 2017-202 - Rev 00
Issue Date : 2023-08-09
Type : Chemical
Substance Number : 2888
Status : Valid

Registration Number : 228MF10183
Registrant's Address : Poli(´)gon Industrial Riera de Caldes Avinguda Cami(´) Reial 51-57 08184 Palau-Solita(´) i Pleamans Barcelona SPAIN
Initial Date of Registration : 2016-09-09
Latest Date of Registration : --

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26210
Submission : 2012-09-27
Status : Inactive
Type : II

Certificate Number : CEP 2018-161 - Rev 02
Issue Date : 2024-01-25
Type : Chemical
Substance Number : 2888
Status : Valid

Date of Issue : 2022-09-01
Valid Till : 2025-07-02
Written Confirmation Number : WC-0074
Address of the Firm :


Date of Issue : 2022-06-08
Valid Till : 2025-07-07
Written Confirmation Number : WC-0055
Address of the Firm :


Date of Issue : 2022-12-16
Valid Till : 2025-12-15
Written Confirmation Number : WC-0544
Address of the Firm :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
 IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
Rupatadine fumarate, Micronised, non-micronised
Certificate Number : CEP 2021-095 - Rev 00
Status : Valid
Issue Date : 2024-07-10
Type : Chemical
Substance Number : 2888
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Certificate Number : CEP 2023-016 - Rev 00
Status : Valid
Issue Date : 2024-07-09
Type : Chemical
Substance Number : 2888
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2018-161 - Rev 02
Status : Valid
Issue Date : 2024-01-25
Type : Chemical
Substance Number : 2888

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2018-213 - Rev 01
Status : Valid
Issue Date : 2022-10-26
Type : Chemical
Substance Number : 2888

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2017-202 - Rev 00
Status : Valid
Issue Date : 2023-08-09
Type : Chemical
Substance Number : 2888

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2018-180 - Rev 02
Status : Valid
Issue Date : 2023-02-02
Type : Chemical
Substance Number : 2888

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : RUPATADINA AUROBINDO
Dosage Form : Tablets
Dosage Strength : 10 mg
Packaging : 30 UNITS 10 MG - ORAL USE
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Spain
Brand Name : Rupafin
Dosage Form : Rupatadina 120Ml 1 Mg/Ml Oral Use
Dosage Strength : os soluz bottle 120 ml 1 mg/ml with graduated syringe
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Spain
Brand Name : Pafinur
Dosage Form : Rupatadina 120Ml 1 Mg/Ml Oral Use
Dosage Strength : os soluz bottle 120 ml 1 mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Spain
Brand Name : Rupafin
Dosage Form : Potion, resolution
Dosage Strength : 1 mg/ml
Packaging : Bottle
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : RUPATADINA MYLAN PHARMA
Dosage Form : Tablets
Dosage Strength : 10 mg
Packaging : 30 UNITS 10 MG - ORAL USE
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Regulatory Info :
Registration Country : Sweden
Brand Name : Pafinur
Dosage Form :
Dosage Strength :
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Spain
Brand Name : Rupatadine Cinfa 10Mg 20 Tablets Efg
Dosage Form : Tablet
Dosage Strength : 10 Mg/Tablet
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Spain
Brand Name : Alergoliber 10Mg 20 Tablets
Dosage Form : Tablet
Dosage Strength : 10 Mg/Tablet
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Spain
Brand Name : Rupafin 1Mg/Ml Oral Solution 1 Bottle Of 120Ml
Dosage Form : Oral Solution
Dosage Strength : 1mg/ml/Oral Solution
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Italy
Brand Name : PAFINUR
Dosage Form : Oral Solution
Dosage Strength : 1 mg/ml
Packaging : 120 ML 1 MG/ML - ORAL USE
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]https://www.pharmacompass.com/radio-compass-blog/top-drugs-and-pharmaceutical-companies-of-2019-by-revenues
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?