NDC Code(s) : 0002-1975-90, 0002-1975-61, 0002-1977-90
Packager : Eli Lilly and Company
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AXIRON testosterone SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| AXIRON testosterone SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
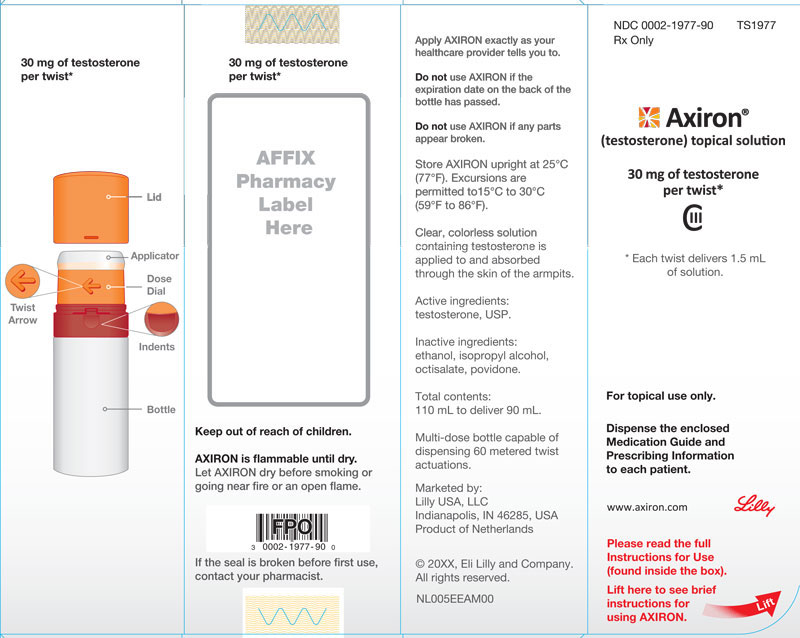
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – Axiron Bottle 30mg
If the seal is broken before first use, contact your pharmacist
NDC 0002-1975-90
TS 1975
Axiron®
(testosterone) topical solution
30 mg of testosterone per pump actuation*
CIII
*Each actuation delivers 1.5 mL of solution
Multi-dose pump capable of dispensing 60 metered pump actuations.
For topical use only with enclosed applicator
Dispense the enclosed Medication Guide to each patient
Rx Only
www.axiron.com
Lilly
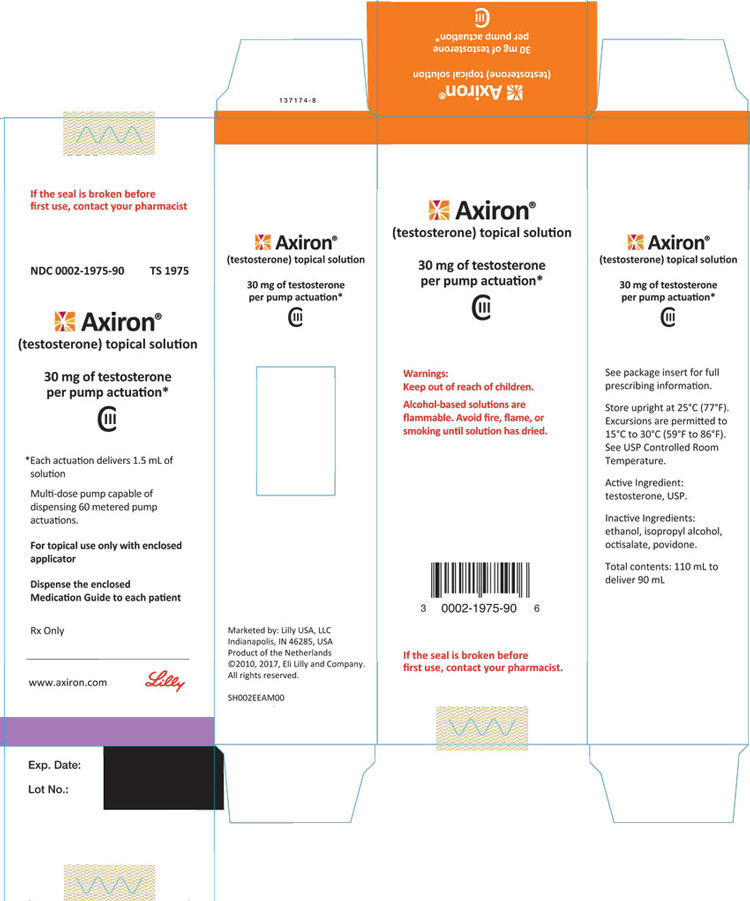
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – Axiron Twist Bottle 30mg 1ct
NDC 0002-1977-90
TS 1977
Rx Only
Axiron®
(testosterone) topical solution
30 mg of testosterone per twist*
CIII
*Each twist delivers 1.5 mL of solution.
For topical use only.
Dispense the enclosed Medication Guide and Prescribing Information to each patient.
www.axiron.com
Lilly
Please read the full Instructions for Use (found inside the box).
Lift here to see brief instructions for using AXIRON.