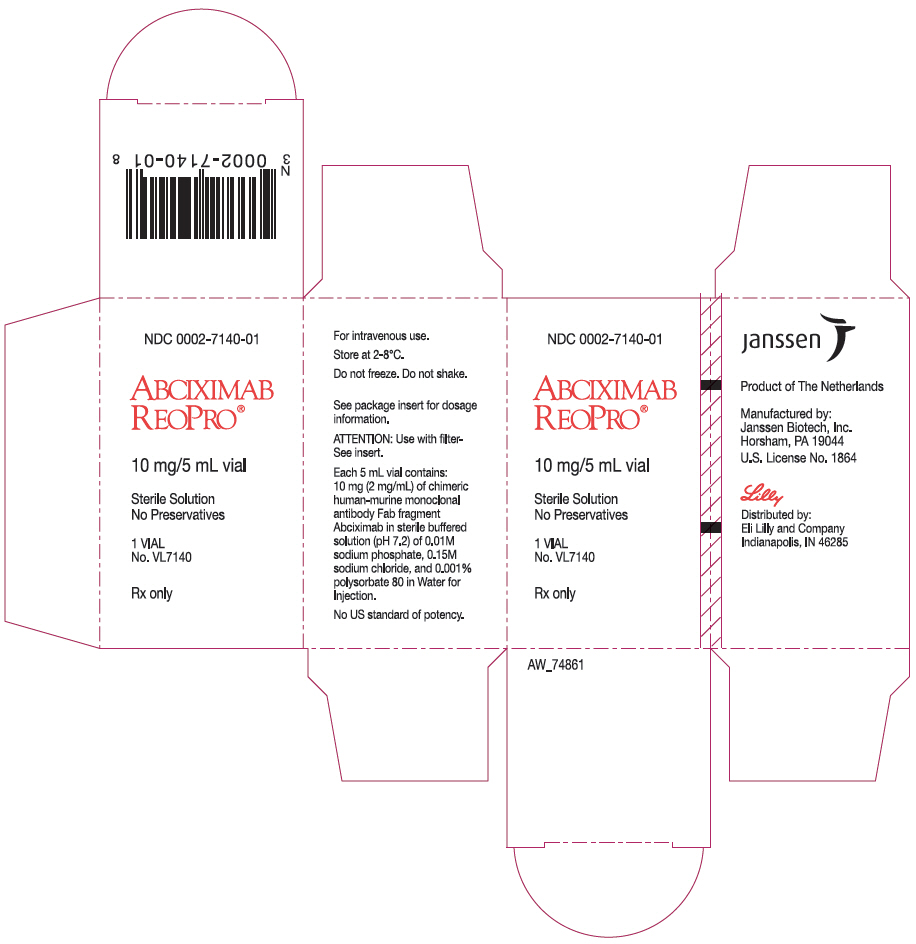
NDC Code(s) : 0002-7140-01
Packager : Eli Lilly and Company
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| REOPROabciximab INJECTION, SOLUTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 0002-7140-01
ABCIXIMAB
REOPRO®
10 mg/5 mL vial
Sterile Solution
No Preservatives
1 VIAL
No. VL7140
Rx only