NDC Code(s) : 0002-7669-01, 0002-7678-01
Packager : Eli Lilly and Company
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CYRAMZA ramucirumab SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CYRAMZA ramucirumab SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Eli Lilly and Company(006421325) |
PRINCIPAL DISPLAY PANEL
PACKAGE LABELING
This section contains a representative sample of product package labeling. Product may be manufactured at other manufacturing sites.
PACKAGE CARTON –CYRAMZA 100 mg/10 mL single-use vial.
NDC 0002-7669-01
Cyramza®
(ramucirumab)
Injection
100 mg/10 mL
(10 mg/mL)
For Intravenous Infusion Only
Must Dilute Prior to Use
Single-Dose Vial
Discard Unused Portion
Keep Refrigerated
Rx only
www.cyramza.com
Lilly
CARTON FOR US ORIGIN
CARTON FOR IRELAND ORIGIN
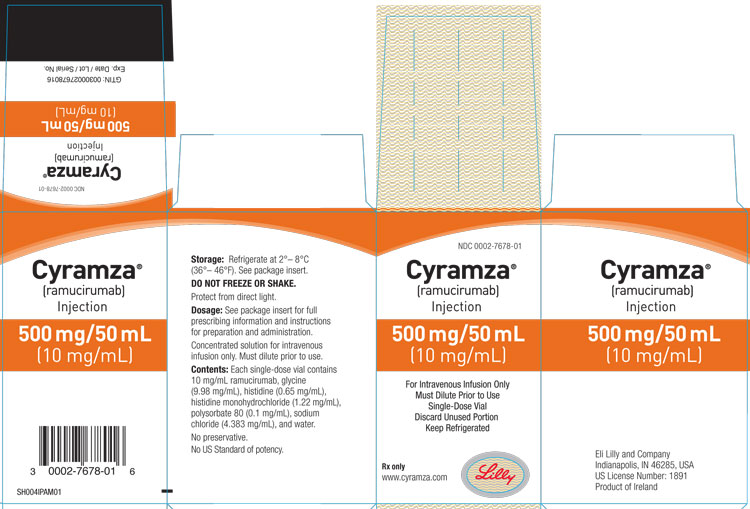
PRINCIPAL DISPLAY PANEL
PACKAGE CARTON – CYRAMZA 500 mg/50mL single-use vial.
NDC 0002-7678-01
Cyramza®
(ramucirumab)
Injection
500 mg/50 mL
(10 mg/mL)
For Intravenous Infusion Only
Must Dilute Prior to Use
Single-Dose Vial
Discard Unused Portion
Keep Refrigerated
Rx only
www.cyramza.com
Lilly
CARTON FOR US ORIGIN
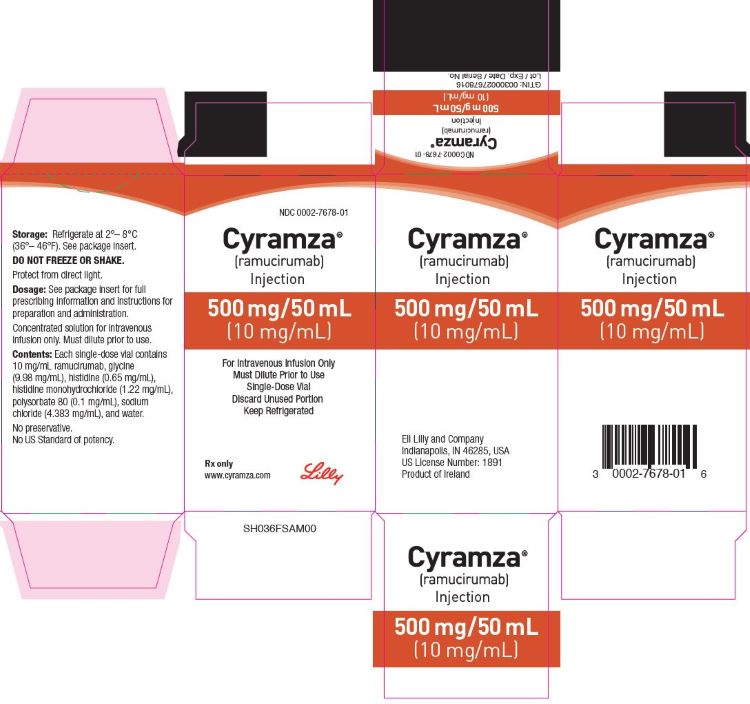
CARTON FOR IRELAND ORIGIN