NDC Code(s) : 0006-5007-01
Packager : Merck Sharp & Dohme LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DELSTRIGOdoravirine, lamivudine, and tenofovir disoproxil fumarate TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LABELER - Merck Sharp & Dohme LLC(118446553) |
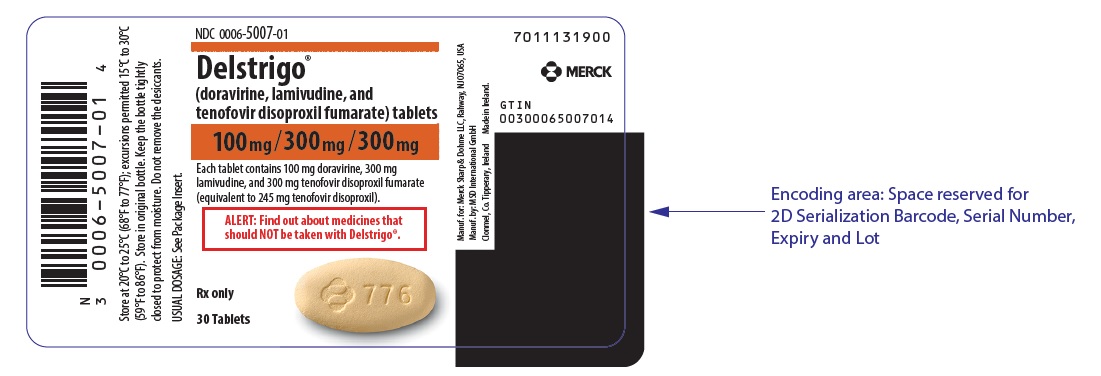
PRINCIPAL DISPLAY PANEL
NDC 0006-5007-01
Delstrigo®
(doravirine, lamivudine, and
tenofovir disoproxil fumarate) tablets
100mg/300mg /300mg
Each tablet contains 100 mg doravirine, 300 mg
lamivudine, and 300 mg tenofovir disoproxil fumarate
(equivalent to 245 mg tenofovir disoproxil).
ALERT: Find out about medicines that
should NOT be taken with Delstrigo®.
Rx only
30 Tablets