NDC Code(s) : 0007-4640-13, 0007-4641-13, 0007-4641-61, 0007-4642-13, 0007-4643-13, 0007-4646-13, 0007-4515-27
Packager : GlaxoSmithKline LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| PROMACTAeltrombopag olamine TABLET, FILM COATED | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| PROMACTAeltrombopag olamine TABLET, FILM COATED | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| PROMACTAeltrombopag olamine TABLET, FILM COATED | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| PROMACTAeltrombopag olamine TABLET, FILM COATED | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| PROMACTAeltrombopag olamine TABLET, FILM COATED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| PROMACTAeltrombopag olamine POWDER, FOR SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
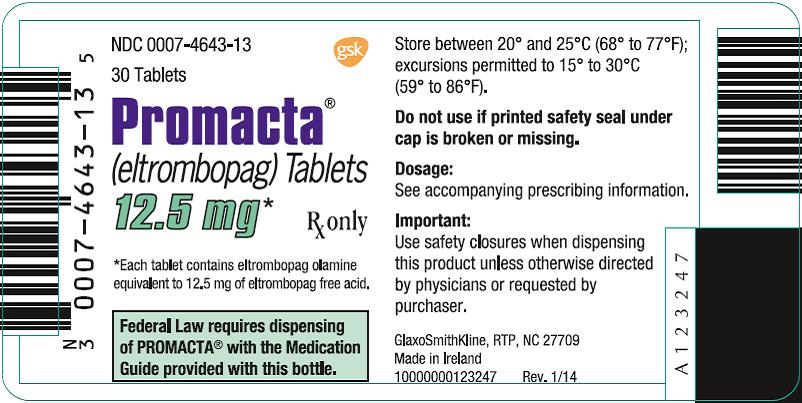
NDC 0007-4643-13
PROMACTA®
(eltrombopag) Tablets
12.5 mg*
30 Tablets
Rx only
*Each tablet contains eltrombopag olamine equivalent to 12.5 mg of eltrombopag free acid.
Federal Law requires dispensing of PROMACTA® with the Medication Guide provided with this bottle.
Store between 20o and 25oC (68o to 77oF); excursions permitted to 15o to 30oC (59o to 86oF).
Do not use if printed safety seal under cap is broken or missing.
Dosage: See accompanying prescribing information.
Important: Use safety closures when dispensing this product unless otherwise directed by physicians or requested by purchaser.
GlaxoSmithKline, RTP, NC 27709
Made in Ireland
- 10000000123247 Rev. 1/14
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0007-4640-13
PROMACTA®
(eltrombopag) Tablets
25 mg*
30 Tablets
Rx only
*Each tablet contains eltrombopag olamine equivalent to 25 mg of eltrombopag free acid.
Federal Law requires dispensing of PROMACTA® with the Medication Guide provided with this bottle.
Store between 20o and 25oC (68o to 77oF); excursions permitted to 15o to 30oC (59o to 86oF).
Do not use if printed safety seal under cap is broken or missing.
Dosage: See accompanying prescribing information.
Important: Use safety closures when dispensing this product unless otherwise directed by physicians or requested by purchaser.
GlaxoSmithKline, RTP, NC 27709
Made in Ireland
- 10000000123416 Rev. 1/14

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0007-4641-13
PROMACTA®
(eltrombopag) Tablets
50 mg*
30 Tablets
Rx only
*Each tablet contains eltrombopag olamine equivalent to 50 mg of eltrombopag free acid.
Federal Law requires dispensing of PROMACTA® with the Medication Guide provided with this bottle.
Store between 20o and 25oC (68o to 77oF); excursions permitted to 15o to 30oC (59o to 86oF).
Do not use if printed safety seal under cap is broken or missing.
Dosage: See accompanying prescribing information.
Important: Use safety closures when dispensing this product unless otherwise directed by physicians or requested by purchaser.
GlaxoSmithKline, RTP, NC 27709
Made in Ireland
- 10000000123417 Rev. 1/14

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0007-4642-13
PROMACTA®
(eltrombopag) Tablets
75 mg*
30 Tablets
Rx only
*Each tablet contains eltrombopag olamine equivalent to 75 mg of eltrombopag free acid.
Federal Law requires dispensing of PROMACTA® with the Medication Guide provided with this bottle.
Store between 20o and 25oC (68o to 77oF); excursions permitted to 15o to 30oC (59o to 86oF).
Do not use if printed safety seal under cap is broken or missing.
Dosage: See accompanying prescribing information.
Important: Use safety closures when dispensing this product unless otherwise directed by physicians or requested by purchaser.
GlaxoSmithKline, RTP, NC 27709
Made in Ireland
- 10000000123248 Rev. 1/14

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0007-4646-13
PROMACTA®
(eltrombopag) Tablets
100 mg*
30 Tablets
Rx only
*Each tablet contains eltrombopag olamine equivalent to 100 mg of eltrombopag free acid.
Federal Law requires dispensing of PROMACTA® with the Medication Guide provided with this bottle.
Store between 20o and 25oC (68o to77oF); excursions permitted to 15o to 30oC (59o to 86oF). Do not remove desiccant.
Do not use if printed safety seal under cap is broken or missing.
Dosage:
See accompanying prescribing information.
Important:
Use safety closures when dispensing this product unless otherwise directed by physicians or requested by purchaser.
GlaxoSmithKline, RTP, NC 27709
Made in Ireland
10000000123249 Rev. 1/14

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
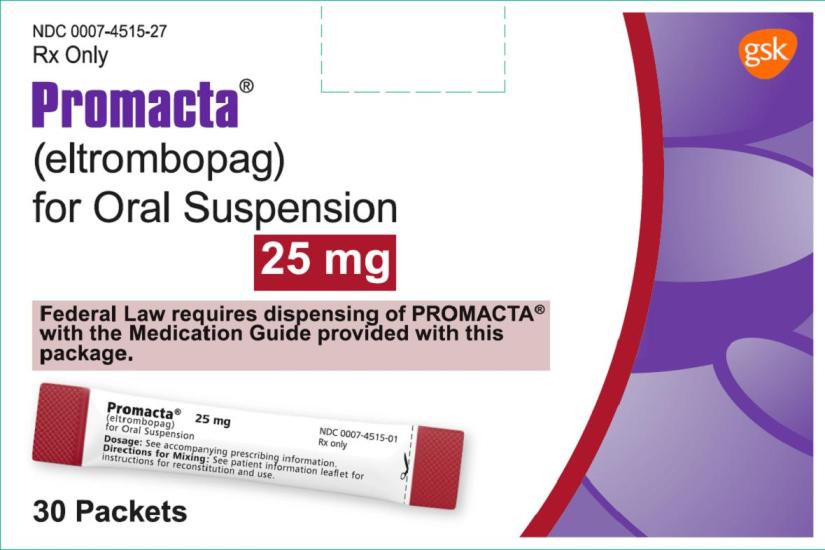
NDC 0007-4514-27
PROMACTA®
(eltrombopag) for Oral Suspension
25 mg
Rx Only
Federal Law requires dispensing of PROMACTA® with the Medication Guide provided with this package.
30 Packets
©2015, the GSK group of companies
- DEVCOMP-0003801 Rev. 8/2015