NDC Code(s) : 0009-0260-01, 0009-0260-04, 0009-0260-17, 0009-0260-02, 0009-0370-03, 0009-0370-05
Packager : Pharmacia & Upjohn Company LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ColestidColestipol Hydrochloride GRANULE, FOR SUSPENSION | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Flavored ColestidColestipol Hydrochloride GRANULE, FOR SUSPENSION | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Pharmacia & Upjohn Company LLC(618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-0260, 0009-0370), API MANUFACTURE(0009-0260, 0009-0370), LABEL(0009-0260, 0009-0370), MANUFACTURE(0009-0260), PACK(0009-0260, 0009-0370) | |
PRINCIPAL DISPLAY PANEL
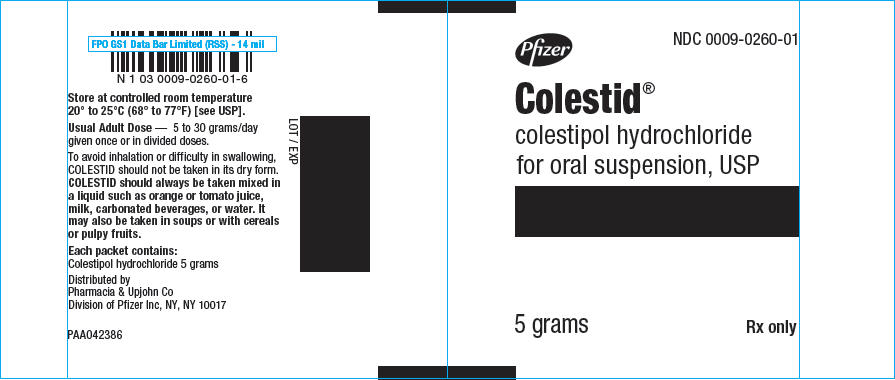
Pfizer
NDC 0009-0260-01
Colestid
®
colestipol hydrochloride
for oral suspension, USP
5 grams
Rx only
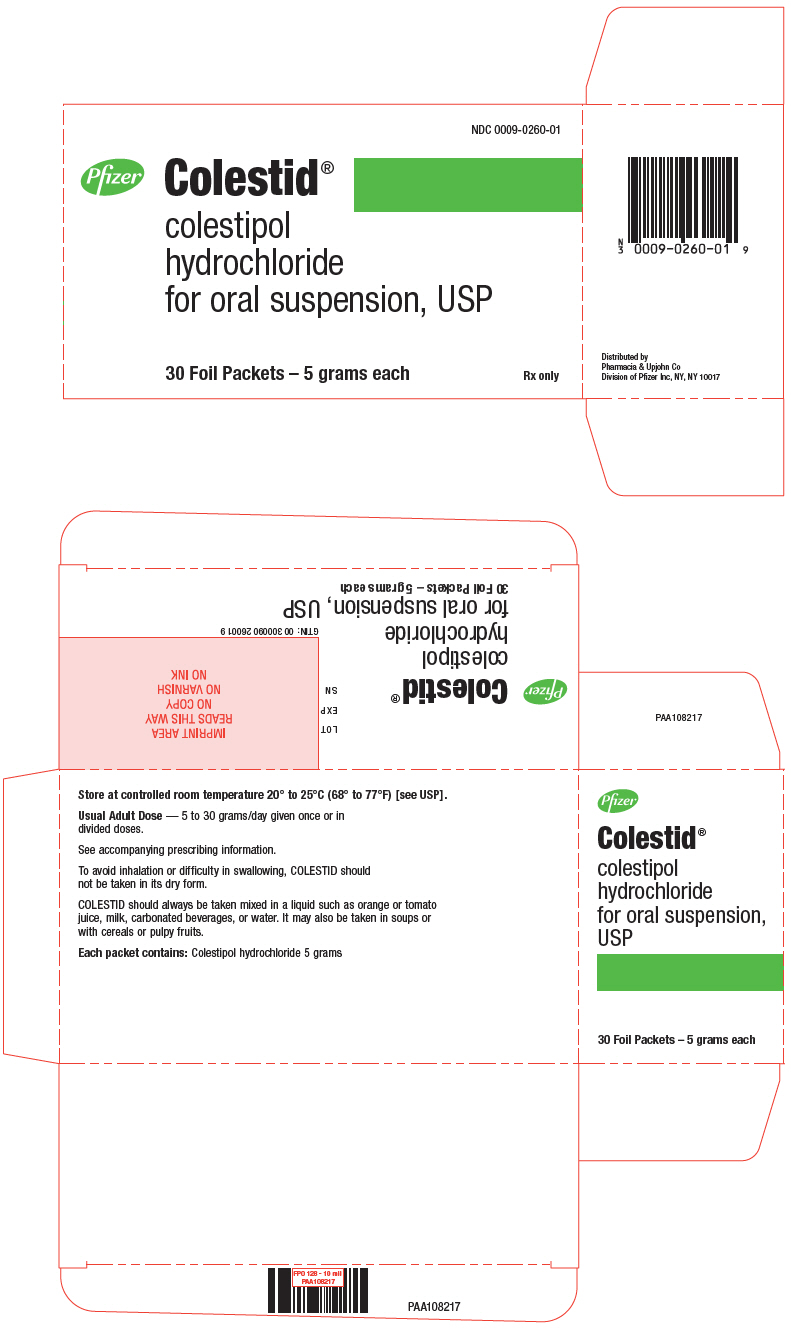
PRINCIPAL DISPLAY PANEL
NDC 0009-0260-01
Pfizer
Colestid
®
colestipol
hydrochloride
for oral suspension, USP
30 Foil Packets – 5 grams each
Rx only
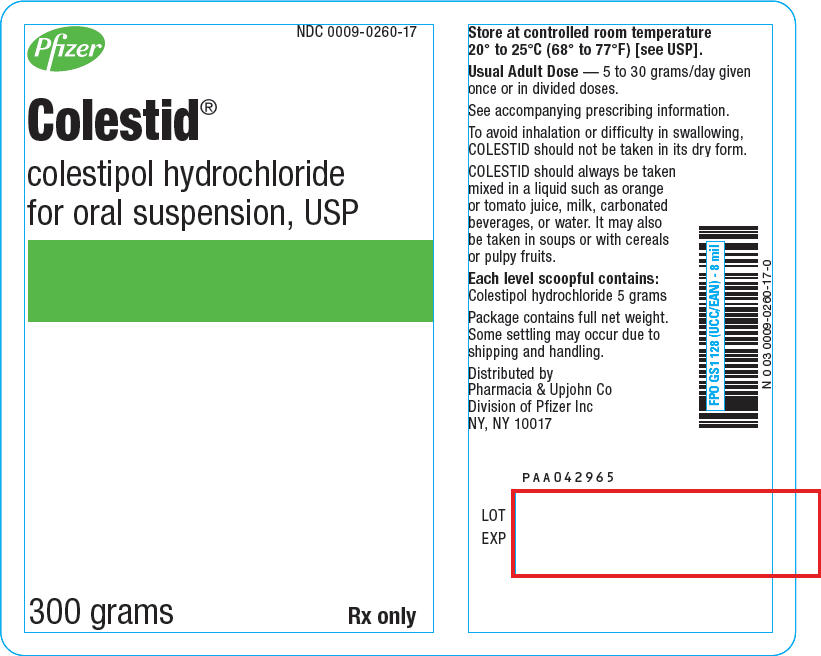
PRINCIPAL DISPLAY PANEL
Pfizer
NDC 0009-0260-17
Colestid ®
colestipol hydrochloride
for oral suspension, USP
300 grams
Rx only
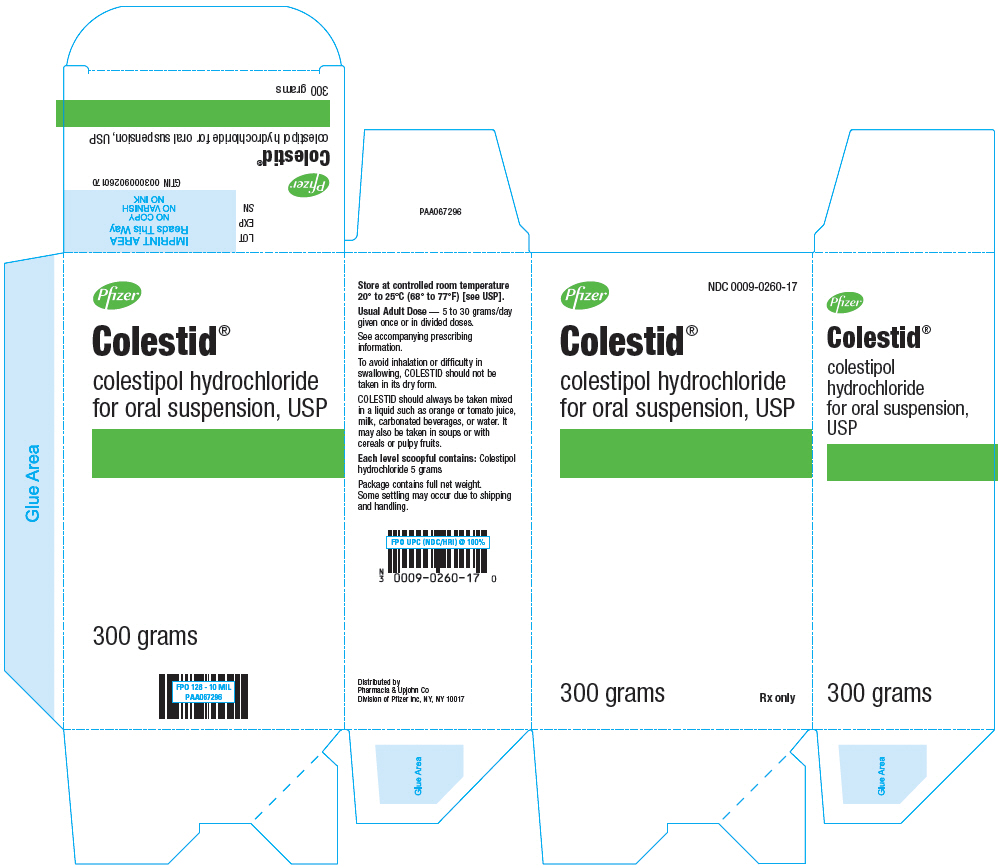
PRINCIPAL DISPLAY PANEL
Pfizer
NDC 0009-0260-17
Colestid ®
colestipol hydrochloride
for oral suspension, USP
300 grams
Rx only
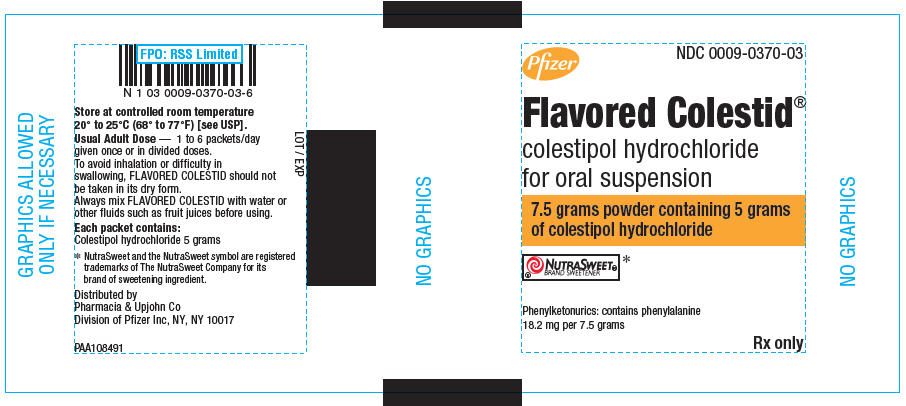
PRINCIPAL DISPLAY PANEL
Pfizer
NDC 0009-0370-03
Flavored Colestid
®
colestipol hydrochloride
for oral suspension
7.5 grams powder containing 5 grams
of colestipol hydrochloride
NUTRASWEET®
BRAND SWEETENER*
Phenylketonurics: contains phenylalanine
18.2 mg per 7.5 grams
Rx only
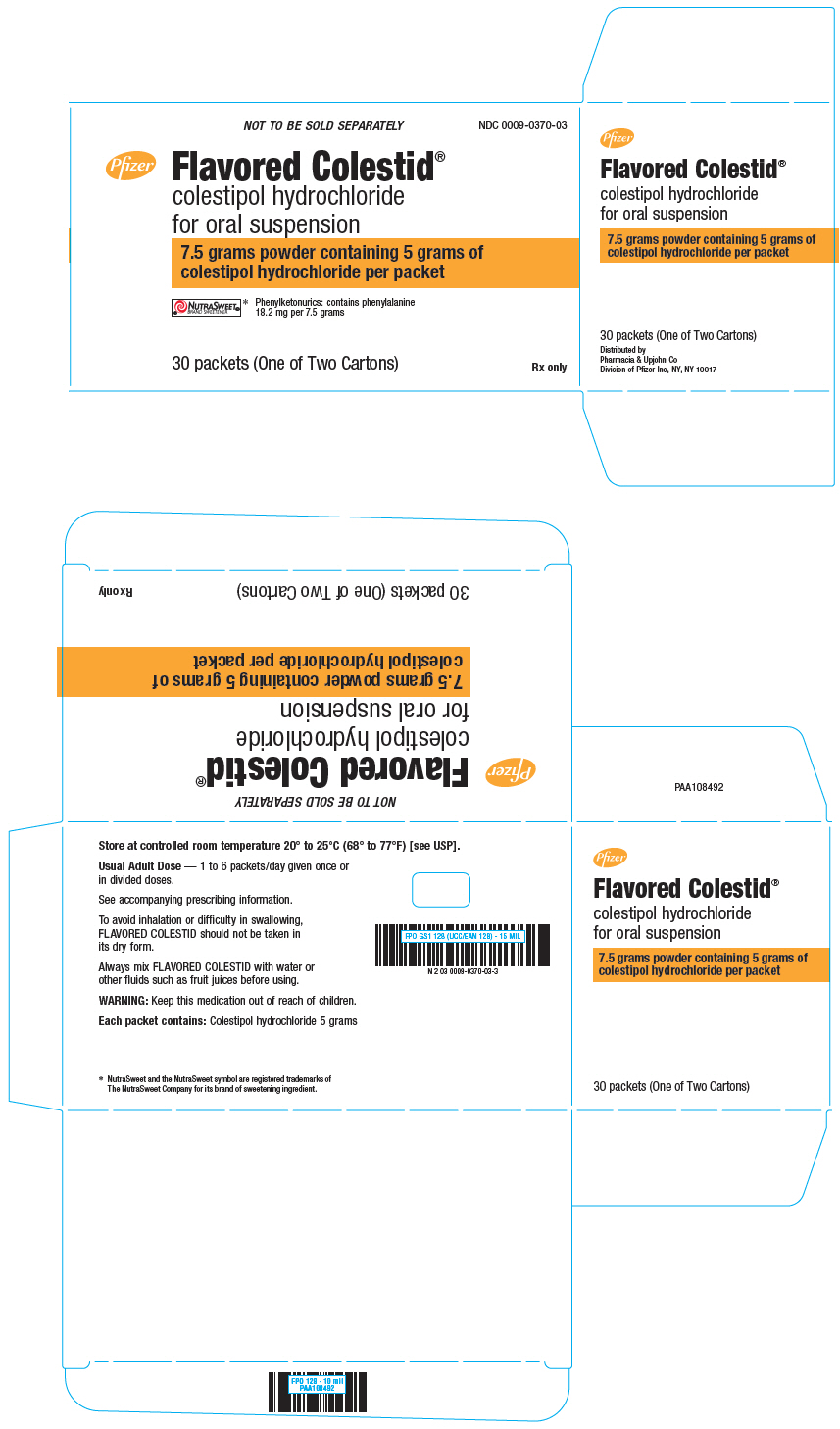
PRINCIPAL DISPLAY PANEL
NOT TO BE SOLD SEPARATELY
NDC 0009-0370-03
Pfizer
Flavored Colestid
®
colestipol hydrochloride
for oral suspension
7.5 grams powder containing 5 grams of
colestipol hydrochloride per packet
NUTRASWEET®
BRAND SWEETENER*
Phenylketonurics: contains phenylalanine
18.2 mg per 7.5 grams
30 packets (One of Two Cartons)
Rx only
PRINCIPAL DISPLAY PANEL
NDC 0009-0370-03
Pfizer
Flavored Colestid
®
colestipol hydrochloride
for oral suspension
7.5 grams powder containing 5 grams of
colestipol hydrochloride per packet
NUTRASWEET®
BRAND SWEETENER*
Phenylketonurics: contains phenylalanine
18.2 mg per 7.5 grams
60 packets
Rx only

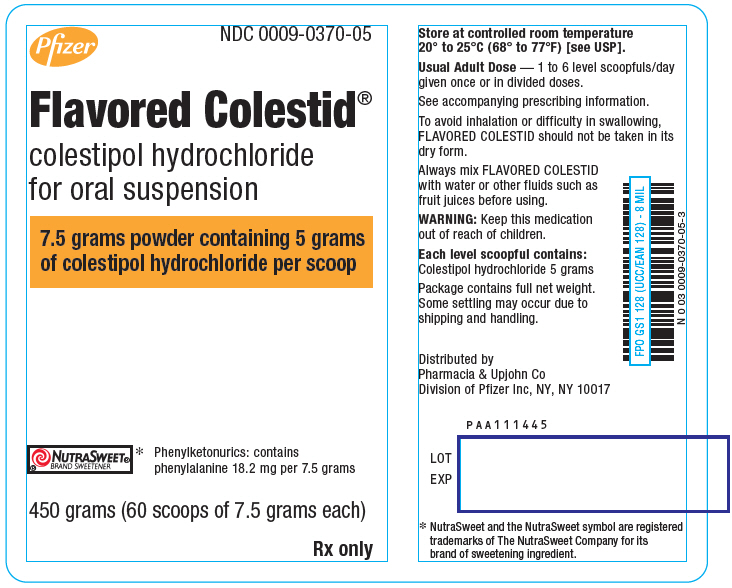
PRINCIPAL DISPLAY PANEL
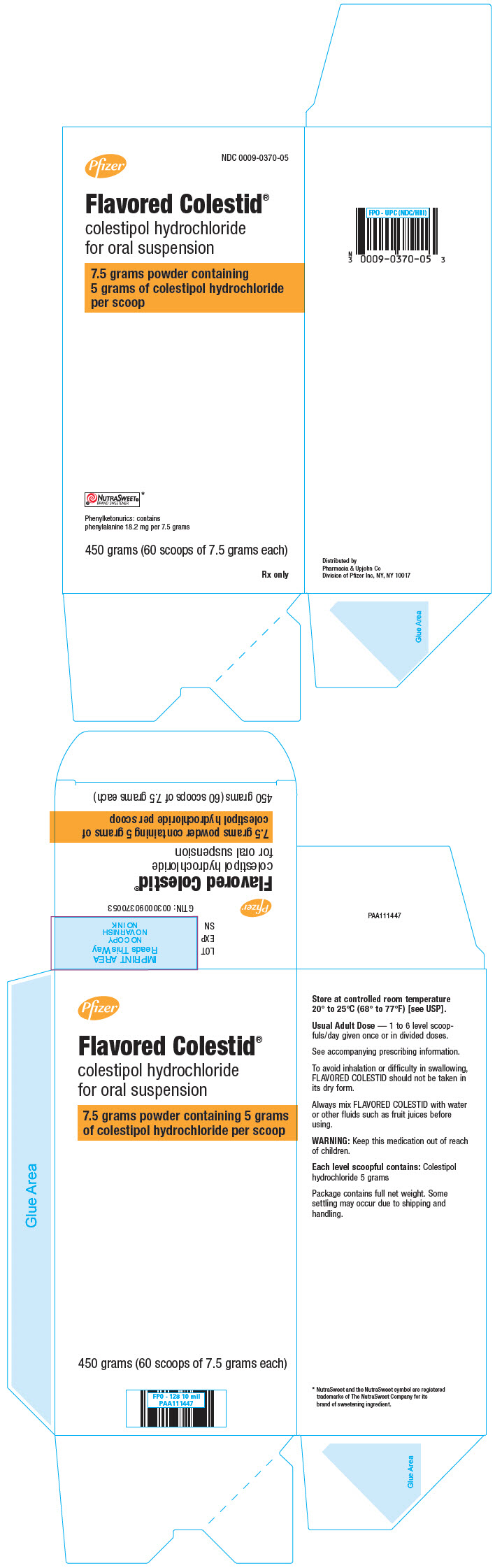
Pfizer
NDC 0009-0370-05
Flavored Colestid
®
colestipol hydrochloride
for oral suspension
7.5 grams powder containing 5 grams
of colestipol hydrochloride per scoop
NUTRASWEET®
BRAND SWEETENER*
Phenylketonurics: contains
phenylalanine 18.2 mg per 7.5 grams
450 grams (60 scoops of 7.5 grams each)
Rx only
PRINCIPAL DISPLAY PANEL
Pfizer
NDC 0009-0370-05
Flavored Colestid
®
colestipol hydrochloride
for oral suspension
7.5 grams powder containing
5 grams of colestipol hydrochloride
per scoop
NUTRASWEET®
BRAND SWEETENER*
Phenylketonurics: contains
phenylalanine 18.2 mg per 7.5 grams
450 grams (60 scoops of 7.5 grams each)
Rx only