NDC Code(s) : 0009-0347-02, 0009-0417-01, 0009-0417-02
Packager : Pharmacia & Upjohn Company LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Depo-Testosteronetestosterone cypionate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Depo-Testosteronetestosterone cypionate INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Pharmacia & Upjohn Company LLC(618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-0347, 0009-0417), API MANUFACTURE(0009-0347, 0009-0417), LABEL(0009-0347, 0009-0417), MANUFACTURE(0009-0347, 0009-0417), PACK(0009-0347, 0009-0417) | |
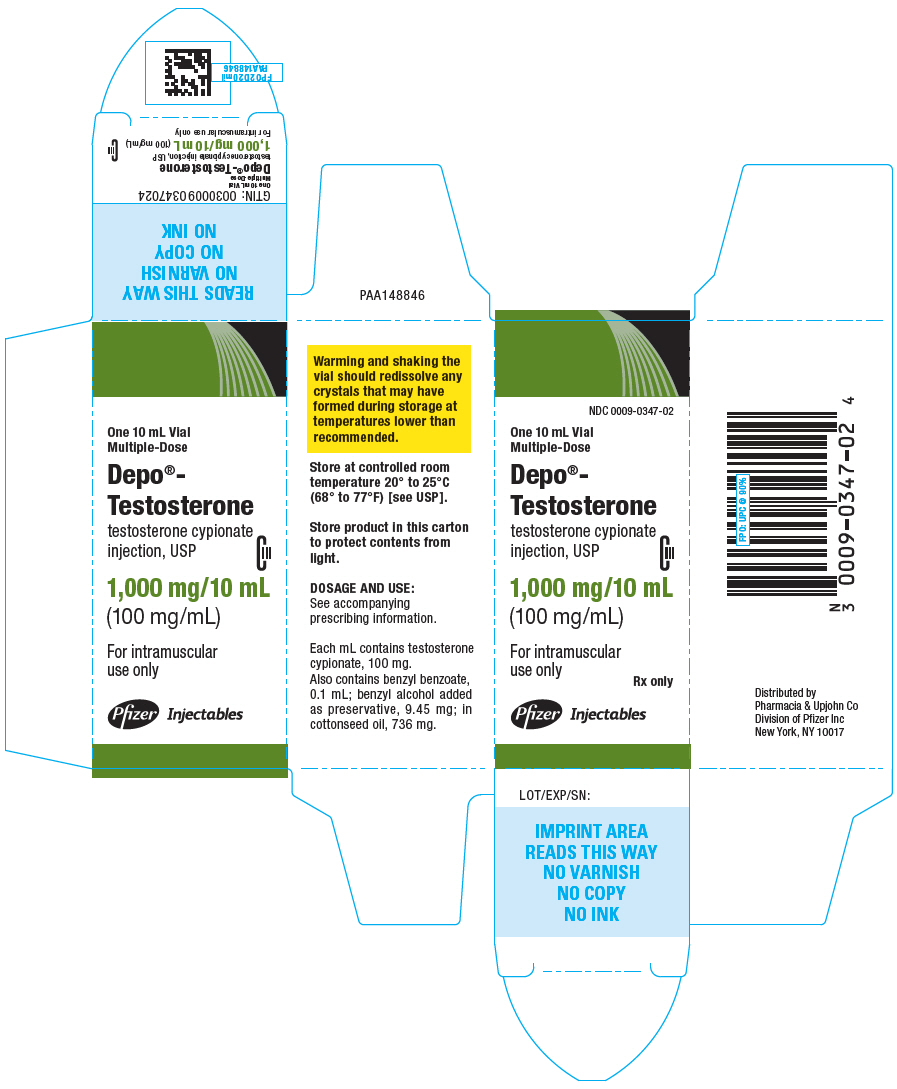
PRINCIPAL DISPLAY PANEL
NDC 0009-0347-02
10 mL Vial
Multiple-Dose
Depo®-Testosterone
testosterone cypionate
injection, USP
CIII
1,000 mg/10 mL
(100 mg/mL)
For intramuscular use only
Rx only
Pfizer Injectables

PRINCIPAL DISPLAY PANEL
NDC 0009-0347-02
One 10 mL Vial
Multiple-Dose
Depo®-
Testosterone
testosterone cypionate
injection, USP
CIII
1,000 mg/10 mL
(100 mg/mL)
For intramuscular
use only
Rx only
Pfizer Injectables
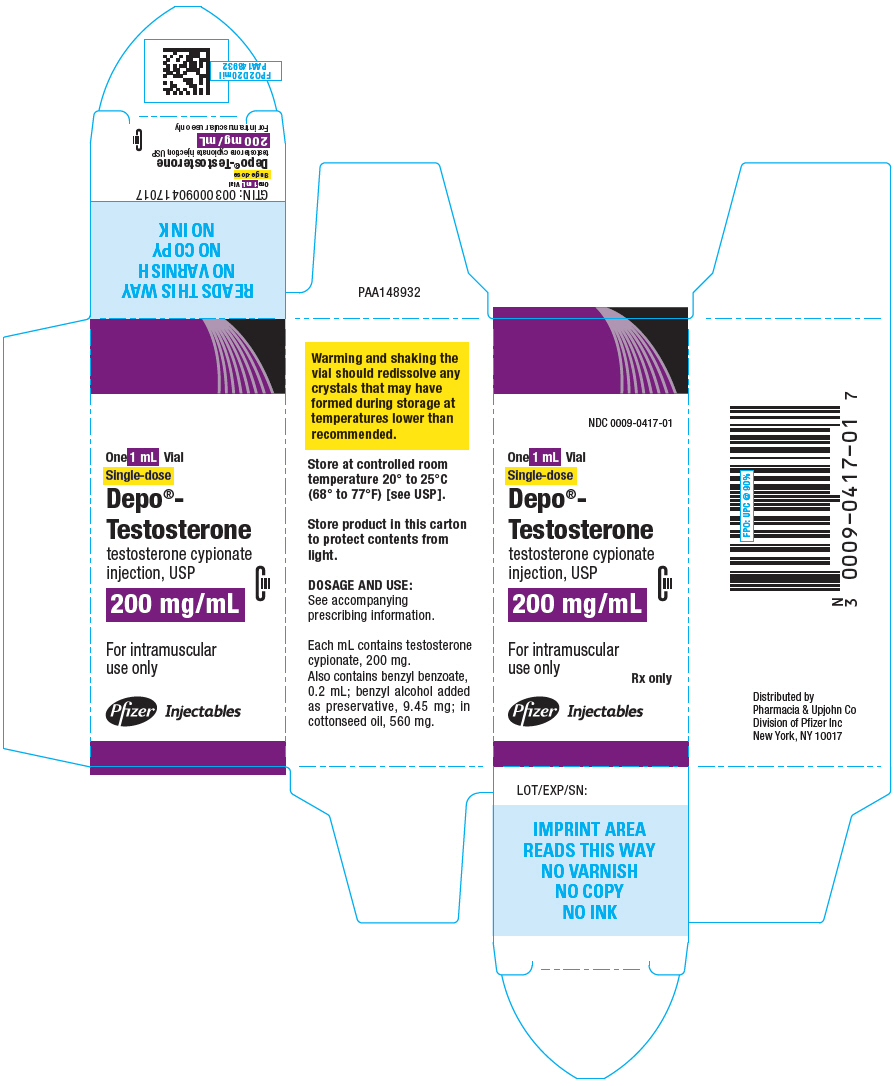
PRINCIPAL DISPLAY PANEL
1 mL Vial
NDC 0009-0417-01
Depo®-
Testosterone
testosterone cypionate
injection, USP
CIII
200 mg/mL
For IM use only
Single-dose
Rx only
Pfizer Injectables

PRINCIPAL DISPLAY PANEL
NDC 0009-0417-01
One 1 mL Vial
Single-dose
Depo®-
Testosterone
testosterone cypionate
injection, USP
CIII
200 mg/mL
For intramuscular
use only
Rx only
Pfizer Injectables