NDC Code(s) : 0025-1001-31, 0025-1041-31, 0025-1031-31
Packager : Pfizer Laboratories Div Pfizer Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Aldactonespironolactone TABLET, FILM COATED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Aldactonespironolactone TABLET, FILM COATED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Aldactonespironolactone TABLET, FILM COATED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LABELER - Pfizer Laboratories Div Pfizer Inc(134489525) |
| REGISTRANT - Pfizer Inc(113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pfizer Inc | 943955690 | ANALYSIS(0025-1001, 0025-1041, 0025-1031) | |
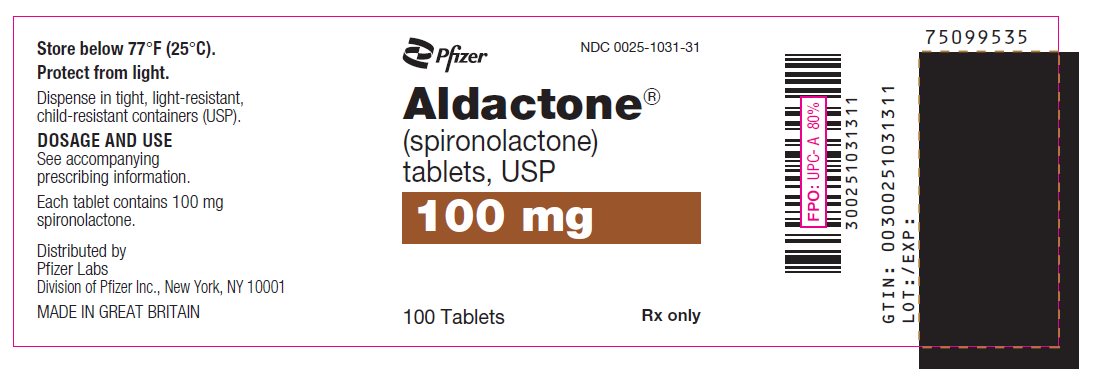
PRINCIPAL DISPLAY PANEL
Pfizer
NDC 0025-1001-31
Aldactone®
(spironolactone)
tablets, USP
25 mg
100 Tablets
Rx only
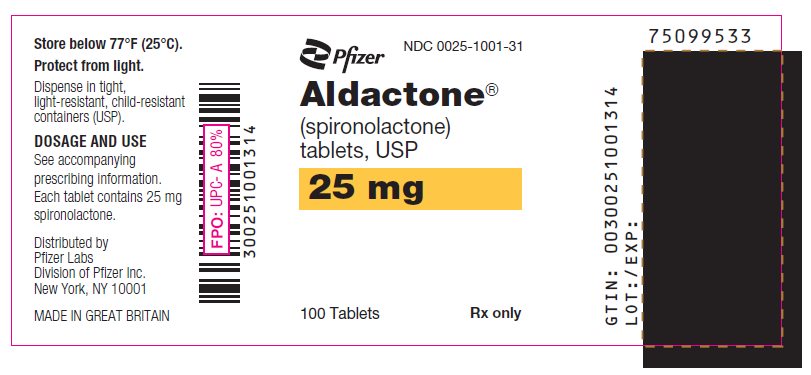
PRINCIPAL DISPLAY PANEL
Pfizer
NDC 0025-1041-31
Aldactone®
(spironolactone)
tablets, USP
50 mg
100 Tablets
Rx only
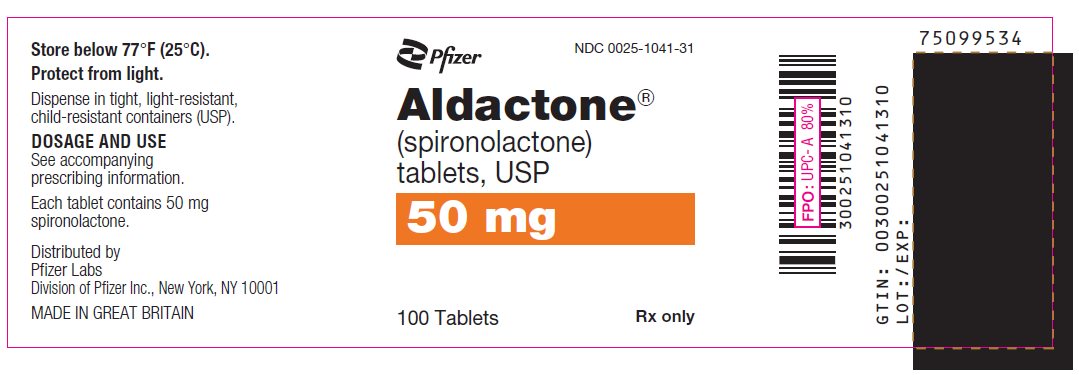
PRINCIPAL DISPLAY PANEL
Pfizer
NDC 0025-1031-31
Aldactone®
(spironolactone)
tablets, USP
100 mg
100 Tablets
Rx only