NDC Code(s) : 0037-6830-15
Packager : Meda Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cortifoamhydrocortisone acetate AEROSOL, FOAM | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - Meda Pharmaceuticals Inc.(051229602) |
PRINCIPAL DISPLAY PANEL
NDC 0037-6830-15
cortifoam®
(hydrocortisone acetate 10%)
rectal foam
Rx Only
15 g net wt
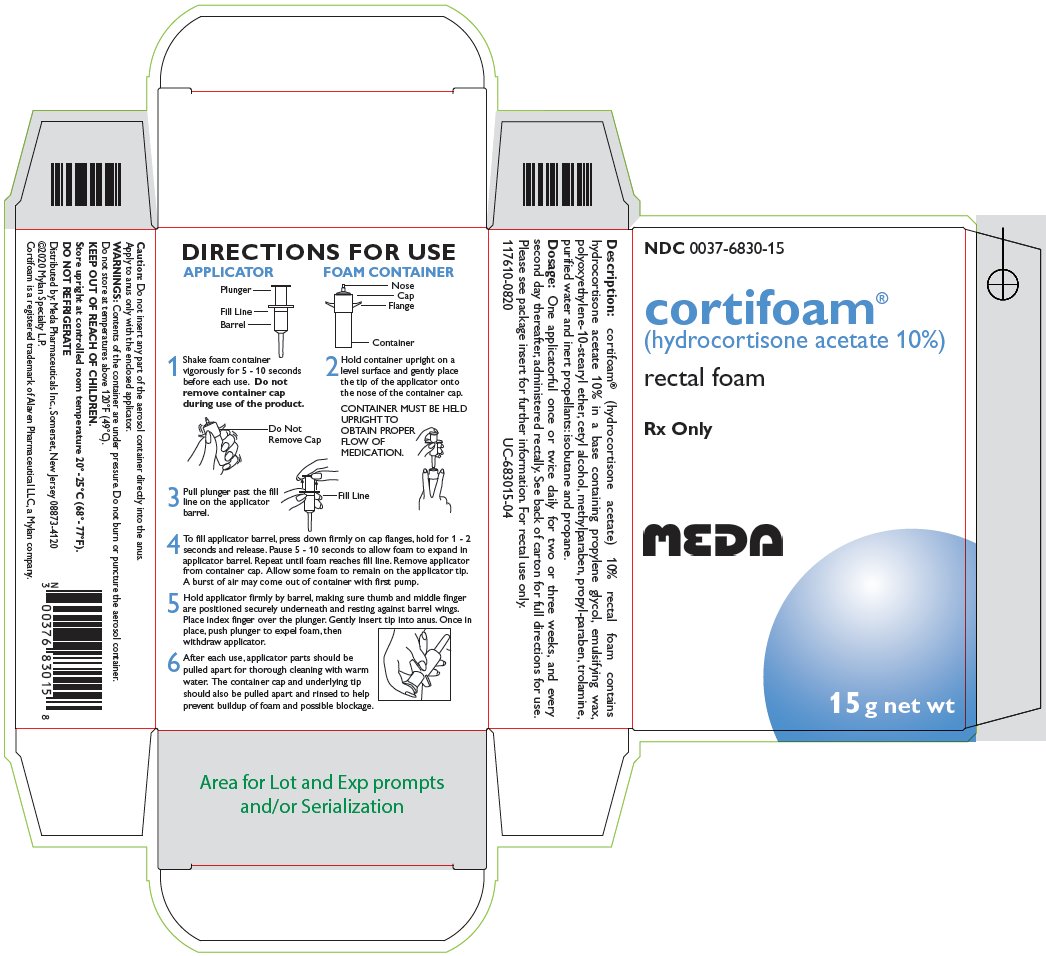
DIRECTIONS FOR USE
|
APPLICATOR |
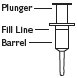
FOAM CONTAINER |
||
 |
 |
||
|
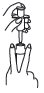
1 Shake foam container vigorously for 5 - 10 seconds before each use. Do not remove container cap during use of the product. |
2 Hold container upright on a level surface and gently place the tip of the applicator onto the nose of the container cap. |
||
 |
CONTAINER MUST BE HELD UPRIGHT TO OBTAIN PROPER FLOW OF MEDICATION. |
||
 |
|||
|
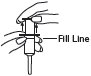
3 Pull plunger past the fill line on the applicator barrel. |
 |
||
|
4 To fill applicator barrel, press down firmly on cap flanges, hold for 1 – 2 seconds and release. Pause 5 - 10 seconds to allow foam to expand in applicator barrel. Repeat until foam reaches fill line. Remove applicator from container cap. Allow some foam to remain on the applicator tip. |
|||
|
5 Hold applicator firmly by barrel, making sure thumb and middle finger are positioned securely underneath and resting against barrel wings. Place index finger over the plunger. Gently insert tip into anus. Once in place, push plunger to expel foam, then withdraw applicator. |
|||
|
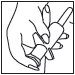
6 After each use, applicator parts should be pulled apart for thorough cleaning with warm water. The container cap and underlying tip should also be pulled apart and rinsed to help prevent buildup of foam and possible blockage. |
 |
||
Description: cortifoam® (hydrocortisone acetate) 10% rectal foam contains hydrocortisone acetate 10% in a base containing propylene glycol, emulsifying wax, polyoxyethylene-10-stearyl ether, cetyl alcohol, methylparaben, propyl-paraben, trolamine, purified water and inert propellants: isobutane and propane.
Dosage: One applicatorful once or twice daily for two or three weeks, and every second day thereafter, administered rectally. See back of carton for full directions for use.
Please see package insert for further information. For rectal use only.
117610-0820 UC-683015-04
Caution: Do not insert any part of the aerosol container directly into the anus. Apply to anus only with the enclosed applicator.
WARNINGS: Contents of the container are under pressure. Do not burn or puncture the aerosol container. Do not store at temperatures above 120°F (49°C).
KEEP OUT OF REACH OF CHILDREN.
Store upright at controlled room temperature 20° - 25°C (68° - 77°F).
DO NOT REFRIGERATE
Distributed by: Meda Pharmaceuticals Inc., Somerset, New Jersey 08873-4120
©2020 Mylan Specialty L.P.
Cortifoam is a registered trademark of Alaven Pharmaceutical LLC, a Mylan company.