NDC Code(s) : 0051-4390-13
Packager : AbbVie Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clorazepate Dipotassium Clorazepate Dipotassium TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
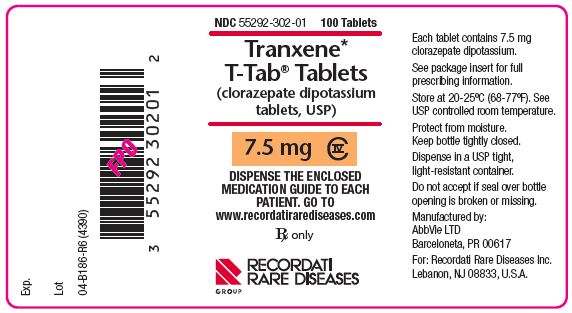
PRINCIPAL DISPLAY PANEL
NDC 55292-302-01
100 Tablets
Tranxene* T-Tab® Tablets
(clorazepate dipotassium tablets, USP)
7.5 mg CIV
DISPENSE THE ENCLOSED MEDICATION GUIDE TO EACH PATIENT. GO TO www.recordatirarediseases.com
Rx only
RECORDATI RARE DISEASES GROUP
Each tablet contains 7.5 mg clorazepate dipotassium.
See package insert for full prescribing information.
Store at 20-25ºC (68-77ºF). See USP controlled room temperature.
Protect from moisture. Keep bottle tightly closed.
Dispense in a USP tight, light-resistant container.
Do not accept if seal over bottle opening is broken or missing.
Manufactured by:
AbbVie LTD
Barceloneta, PR 00617
For: Recordati Rare Diseases Inc.
Lebanon, NJ 08833, U.S.A.
Exp.
Lot
04-B186-R6 (4390)