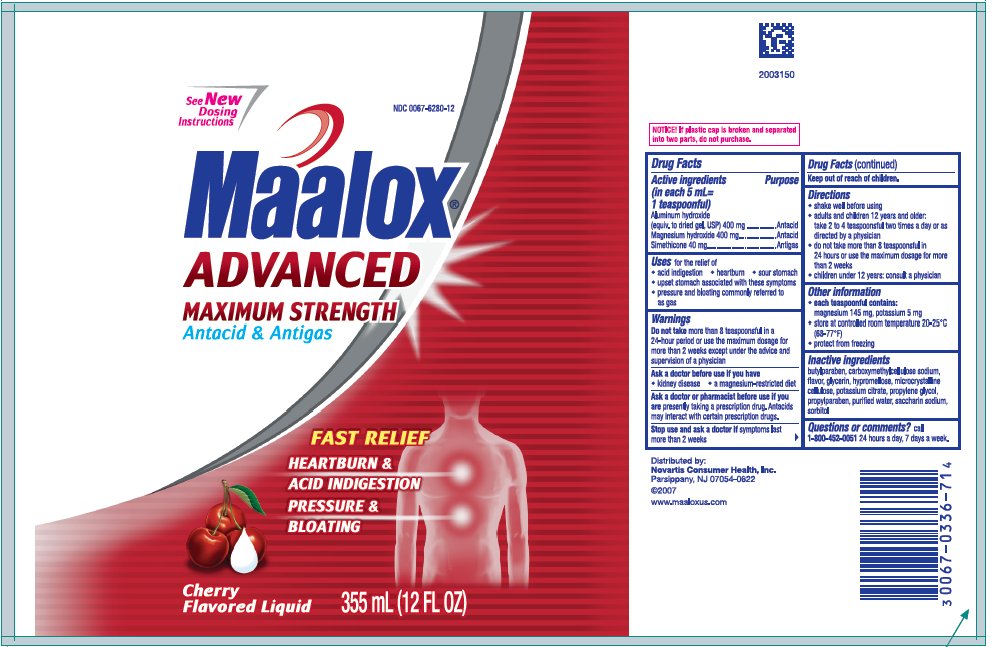
NDC Code(s) : 0067-6280-12, 0067-6280-26
Packager : Novartis Consumer Health, Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Maalox Aluminum Hydroxide, Magnesium Hydroxide,Simethicone LIQUID | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
0067-6280-12
See New Dosing Directions
Maalox
Advanced
Maximum Strength
Antacid &
Antigas
FAST RELIEF
HEARTBURN & ACID INDIGESTION
PRESSURE & BLOATING
Cherry
Flavored Liquid
355 mL (12 FL OZ)