NDC Code(s) : 0074-6633-22, 0074-6633-30
Packager : AbbVie Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| NorvirRitonavir CAPSULE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
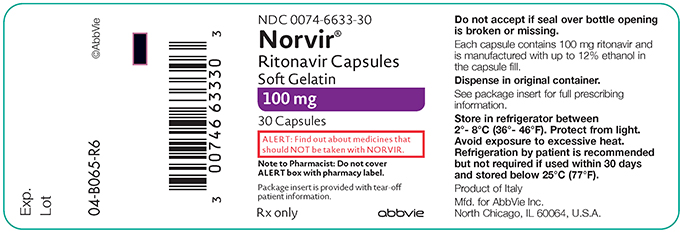
PRINCIPAL DISPLAY PANEL
NDC 0074–6633–30
Norvir®
Ritonavir Capsules Soft Gelatin 100 mg 30 Capsules
ALERT: Find out about medicines that should NOT be taken with NORVIR
Note to Pharmacist: Do not cover ALERT box with pharmacy label.
Package insert is provided with tear-off patient information.
Rx only abbvie