NDC Code(s) : 0075-2451-01, 0075-2451-53
Packager : sanofi-aventis U.S. LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DDAVPdesmopressin acetate SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
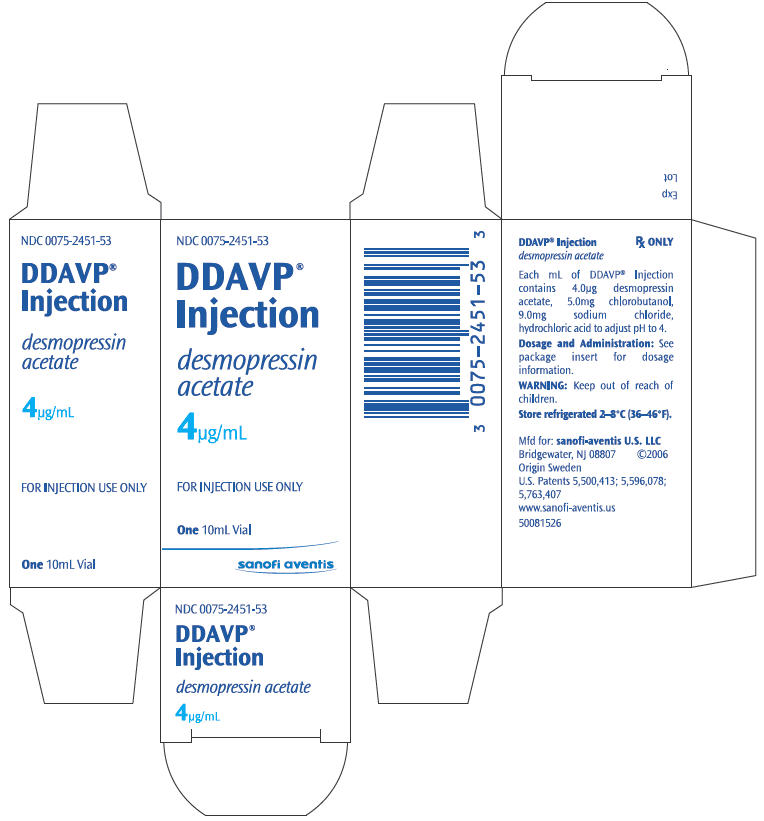
PRINCIPAL DISPLAY PANEL
NDC 0075-2451-53
DDAVP®
Injection
desmopressin
acetate
4µg/mL
FOR INJECTION USE ONLY
One 10mL Vial
sanofi aventis