NDC Code(s) : 0078-0639-41, 0078-0639-68, 0078-0639-98, 0078-0639-97, 0078-1056-97, 0078-1056-99, 0078-1070-68, 0078-1070-96, 0078-1168-61
Packager : Novartis Pharmaceuticals Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| COSENTYXsecukinumab INJECTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| COSENTYXsecukinumab INJECTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| COSENTYXsecukinumab INJECTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| COSENTYXsecukinumab INJECTION, SOLUTION, CONCENTRATE | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LABELER - Novartis Pharmaceuticals Corporation(002147023) |
PRINCIPAL DISPLAY PANEL
NDC 0078-0639-97
Rx only
Cosentyx®
(secukinumab)
Injection
Single-dose Prefilled Syringe
150 mg/mL
1 Prefilled Syringe
ATTENTION: Dispense with enclosed Medication Guide.
For Subcutaneous Use Only
Sterile Solution - Contains No Preservative
Caution: Contains Natural Rubber Latex Which May Cause Allergic Reaction.
NOVARTIS

PRINCIPAL DISPLAY PANEL
NDC 0078-1056-97
Rx only
Cosentyx®
(secukinumab)
Injection
75 mg/0.5 mL
1 Single-dose Prefilled Syringe
For Subcutaneous Use Only
Caution: Contains Natural Rubber Latex
Which May Cause Allergic Reaction.
ATTENTION:
Dispense with enclosed Medication Guide.
NOVARTIS

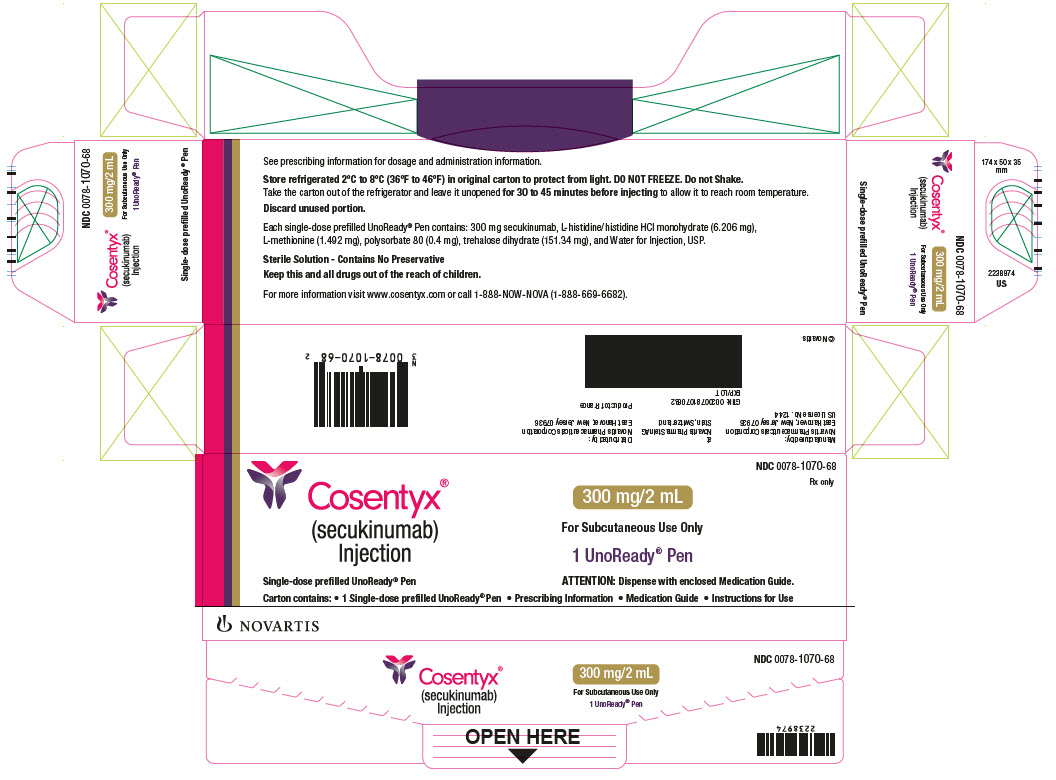
PRINCIPAL DISPLAY PANEL
NDC 0078-1070-68
Rx only
Cosentyx®
(secukinumab)
Injection
Single-dose prefilled UnoReady® Pen
300 mg/2 mL
For Subcutaneous Use Only
1 UnoReady® Pen
ATTENTION: Dispense with enclosed Medication Guide.
Carton contains: • 1 Single-dose prefilled UnoReady® Pen • Prescribing Information • Medication Guide • Instructions for Use
NOVARTIS
PRINCIPAL DISPLAY PANEL
NDC 0078-1168-61
Rx only
Cosentyx®
(secukinumab)
Injection
125 mg/5 mL
(25 mg/mL)
For Intravenous Infusion After Dilution.
ATTENTION: Dispense with enclosed Medication Guide.
1 Single-Dose Vial.
Discard Unused Portion.
NOVARTIS










