NDC Code(s) : 0093-9018-19, 0093-9018-65, 0093-9019-19, 0093-9019-65, 0093-9020-19, 0093-9020-65
Packager : Teva Pharmaceuticals USA, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cyclosporine Cyclosporine CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Cyclosporine Cyclosporine CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Cyclosporine Cyclosporine CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LABELER - Teva Pharmaceuticals USA, Inc.(001627975) |
PRINCIPAL DISPLAY PANEL
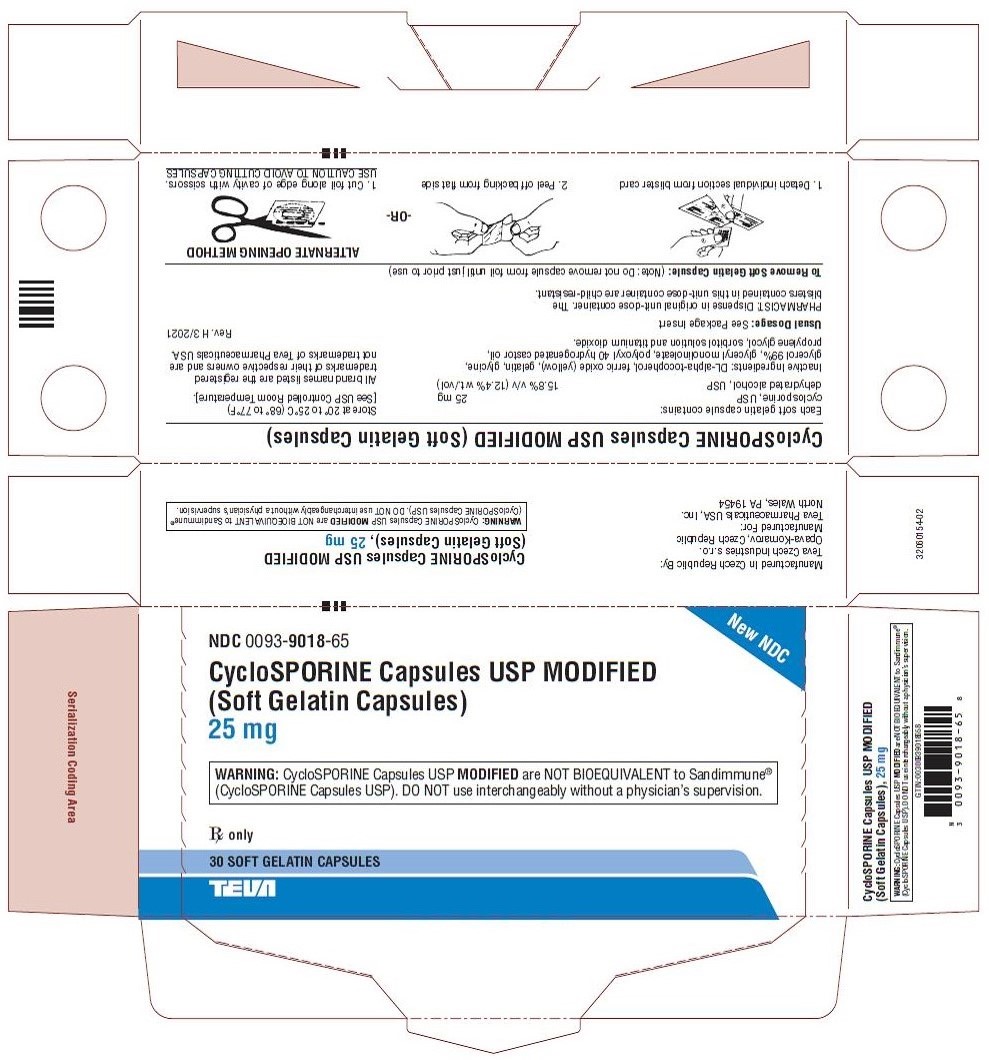
NDC 0093-9018-65
New NDC
CycloSPORINE Capsules USP MODIFIED
(Soft Gelatin Capsules)
25 mg
WARNING: CycloSPORINE Capsules USP MODIFIED are NOT BIOEQUIVALENT to Sandimmune®
(CycloSPORINE Capsules USP). DO NOT use interchangeably without a physician’s supervision.
Rx only
30 SOFT GELATIN CAPSULES
PRINCIPAL DISPLAY PANEL
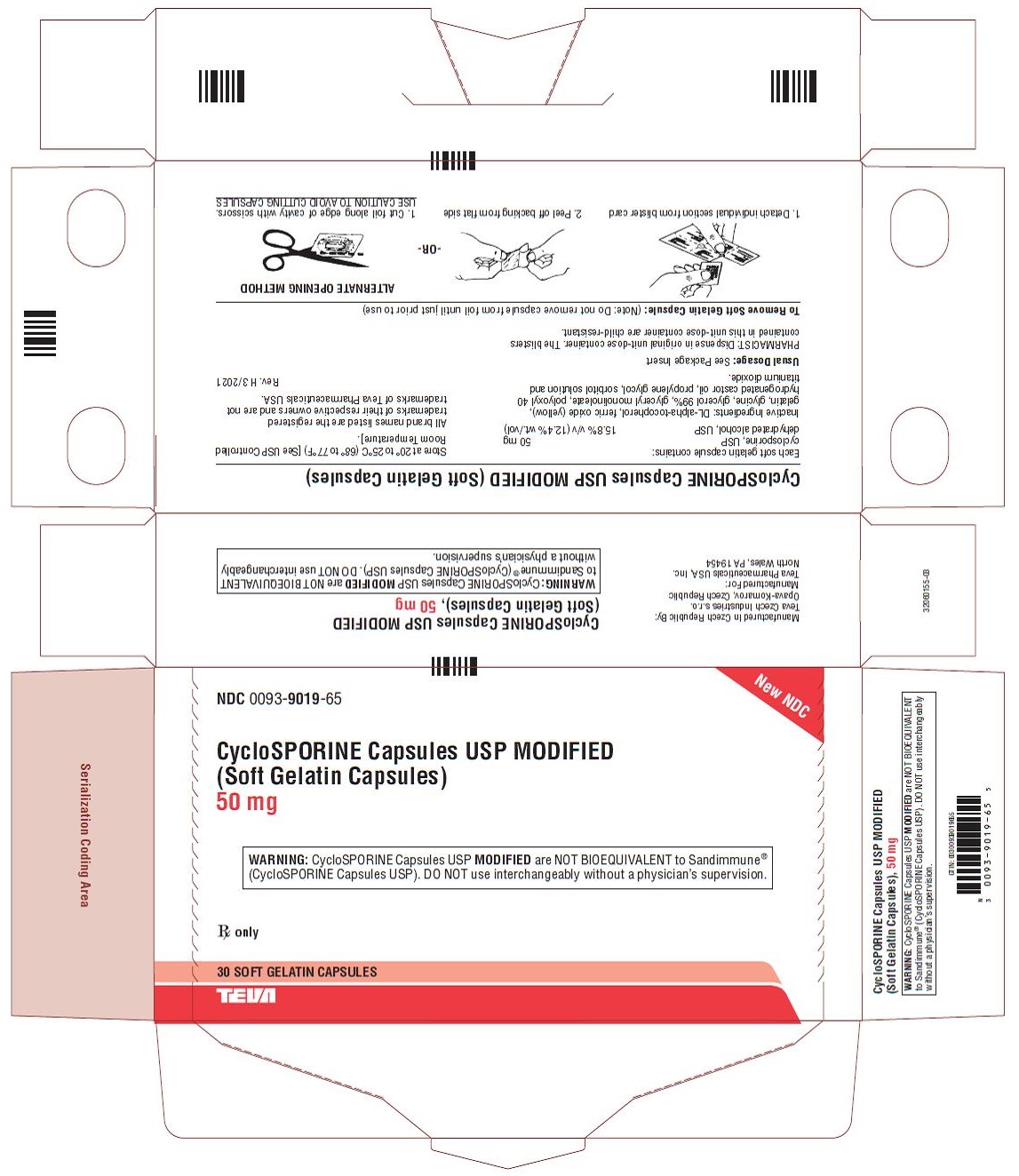
NDC 0093-9019-65
New NDC
CycloSPORINE Capsules USP MODIFIED
(Soft Gelatin Capsules)
50 mg
WARNING: CycloSPORINE Capsules USP MODIFIED are NOT BIOEQUIVALENT to Sandimmune®
(CycloSPORINE Capsules USP). DO NOT use interchangeably without a physician’s supervision.
Rx only
30 SOFT GELATIN CAPSULES
PRINCIPAL DISPLAY PANEL
NDC 0093-9020-65
New NDC
CycloSPORINE Capsules USP MODIFIED
(Soft Gelatin Capsules)
100 mg
WARNING: CycloSPORINE Capsules USP MODIFIED are NOT BIOEQUIVALENT to Sandimmune®
(CycloSPORINE Capsules USP). DO NOT use interchangeably without a physician’s supervision.
Rx only
30 SOFT GELATIN CAPSULES










