NDC Code(s) : 0115-9922-01, 0115-9920-01, 0115-9919-01, 0115-9918-01, 0115-9921-01
Packager : Amneal Pharmaceuticals of New York LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dexmethylphenidate hydrochlorideDexmethylphenidate hydrochloride CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Dexmethylphenidate hydrochlorideDexmethylphenidate hydrochloride CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Dexmethylphenidate hydrochlorideDexmethylphenidate hydrochloride CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Dexmethylphenidate hydrochlorideDexmethylphenidate hydrochloride CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Dexmethylphenidate hydrochlorideDexmethylphenidate hydrochloride CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| LABELER - Amneal Pharmaceuticals of New York LLC(123797875) |
PRINCIPAL DISPLAY PANEL
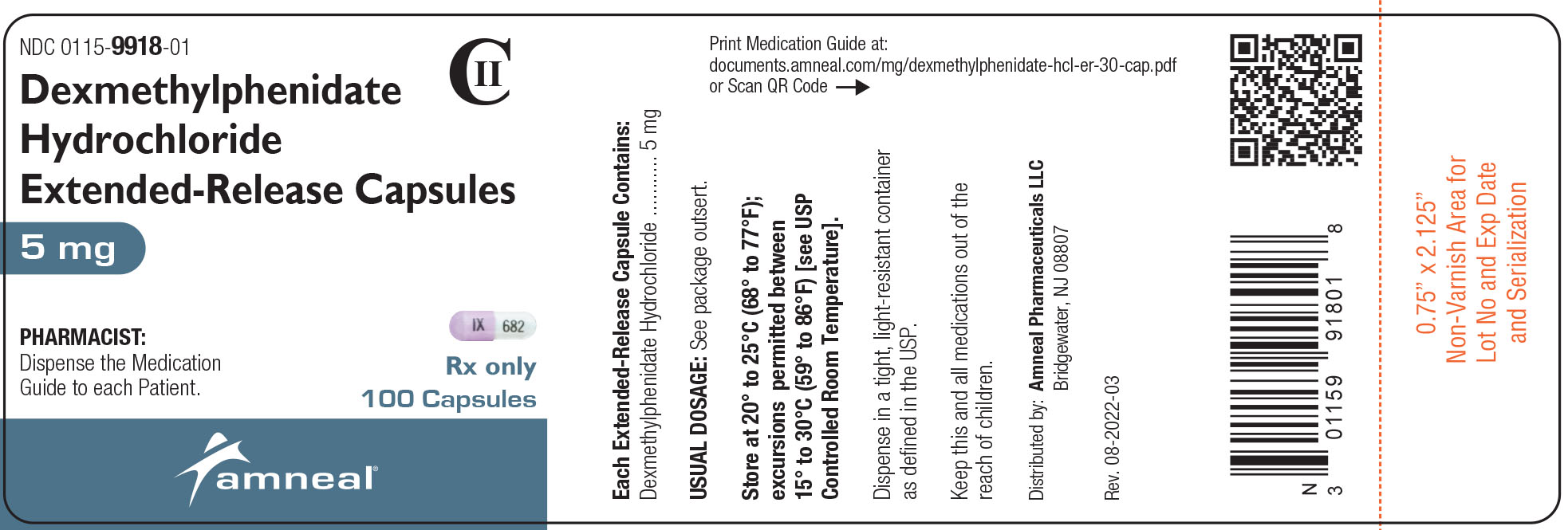
NDC 0115-9918-01
CII
Dexmethylphenidate HCl Extended-Release Capsules, 5 mg
Rx only
100 Capsules
PRINCIPAL DISPLAY PANEL
NDC 0115-9919-01
CII
Dexmethylphenidate HCl Extended-Release Capsules, 10 mg
Rx only
100 Capsules

PRINCIPAL DISPLAY PANEL
NDC 0115-9920-01
CII
Dexmethylphenidate HCl Extended-Release Capsules, 15 mg
Rx only
100 Capsules

PRINCIPAL DISPLAY PANEL
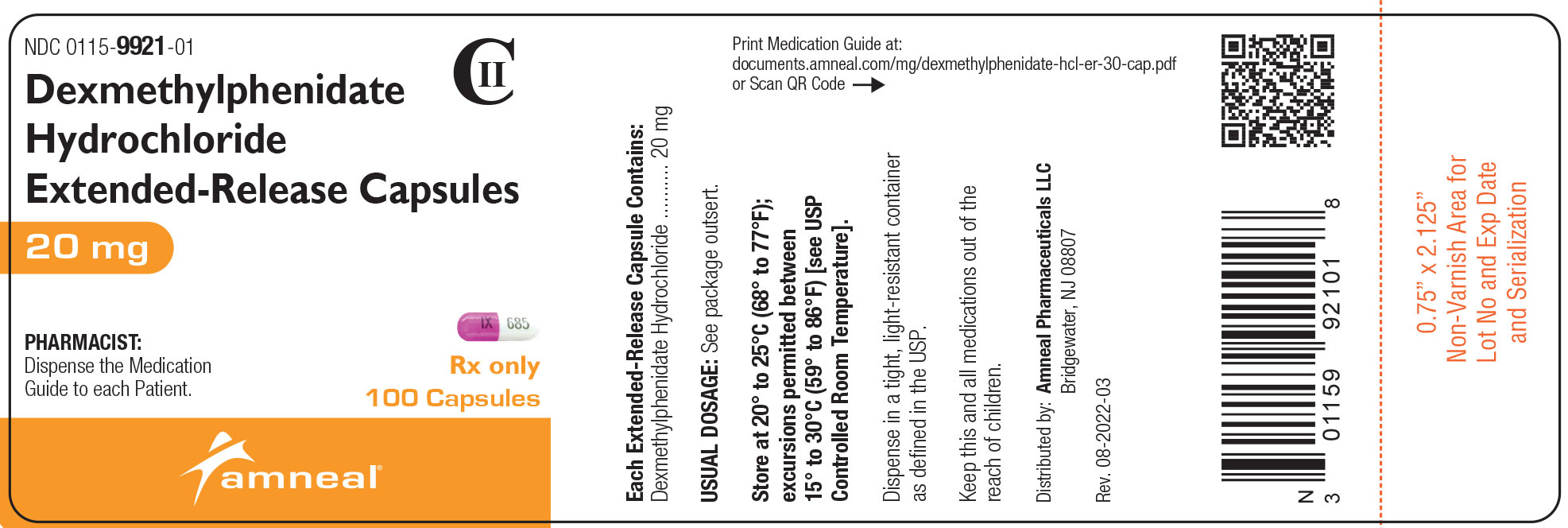
NDC 0115-9921-01
CII
Dexmethylphenidate HCl Extended-Release Capsules, 20 mg
Rx only
100 Capsules
PRINCIPAL DISPLAY PANEL
NDC 0115-9922-01
CII
Dexmethylphenidate HCl Extended-Release Capsules, 30 mg
Rx only
100 Capsules










