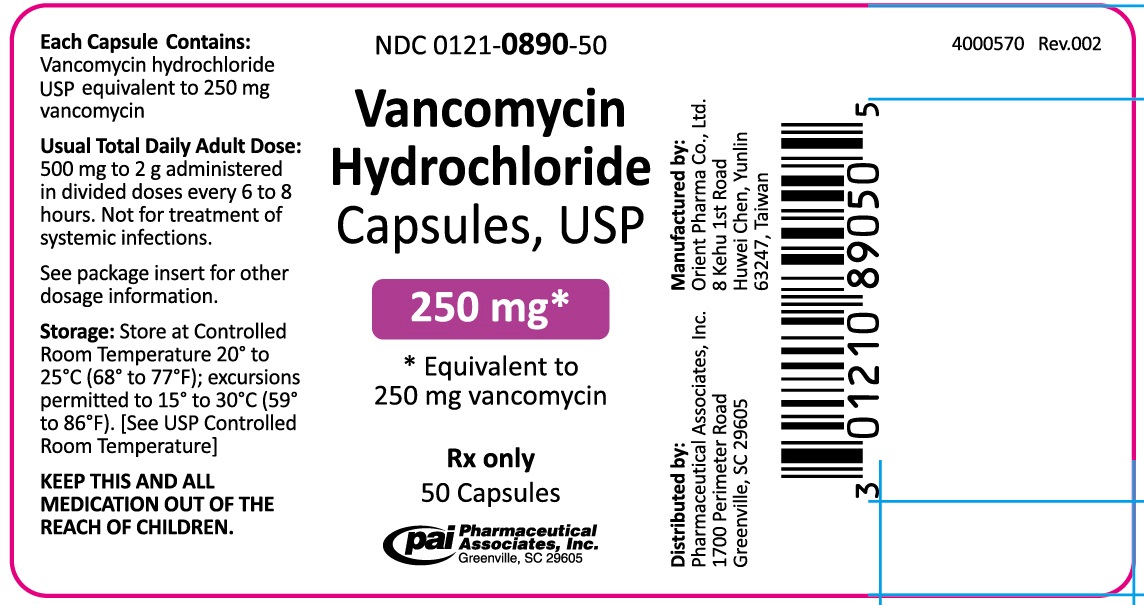
NDC Code(s) : 0121-0867-50, 0121-0890-50
Packager : PAI Holdings, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| VANCOMYCIN HYDROCHLORIDEVANCOMYCIN HYDROCHLORIDE CAPSULE | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| VANCOMYCIN HYDROCHLORIDEVANCOMYCIN HYDROCHLORIDE CAPSULE | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LABELER - PAI Holdings, LLC(044940096) |
PRINCIPAL DISPLAY PANEL
Vancomycin Hydrochloride Capsules USP, Equiv. to 125 mg vancomycin
NDC 0121-0867-50, Rx Only 50 Capsules

Vancomycin Hydrochloride Capsules USP, Equiv. to 250 mg vancomycin
NDC 0121-0890-50, Rx Only 50 Capsules