NDC Code(s) : 0169-2911-15, 0169-2911-90, 0169-2911-97
Packager : Novo Nordisk
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Xultophy 100/3.6(insulin degludec and liraglutide) INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Novo Nordisk(622920320) |
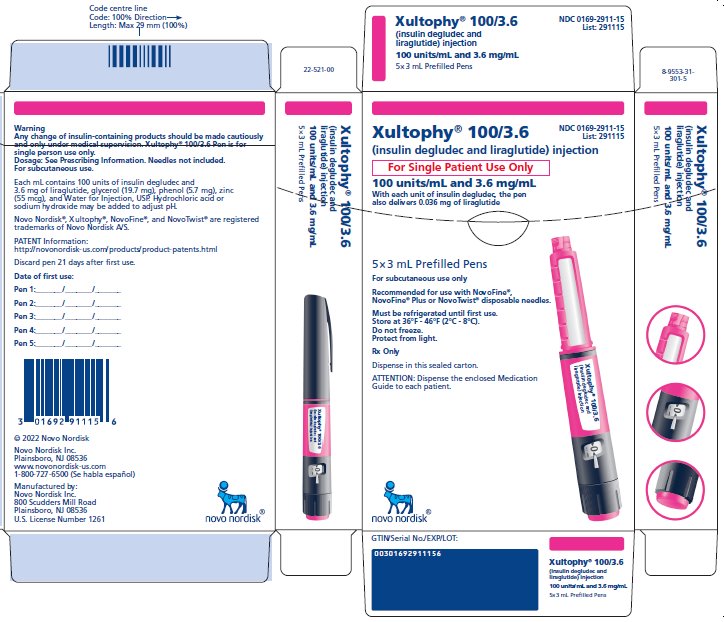
PRINCIPAL DISPLAY PANEL
Xultophy® 100/3.6
NDC 0169-2911-15
List: 291115
(insulin degludec and liraglutide) injection
For Single Patient Use Only
100 units/mL and 3.6 mg/mL
With each unit of insulin degludec, the pen
also delivers 0.036 mg of liraglutide
5x3 mL Prefilled Pens
For subcutaneous use only
Recommended for use with NovoFine®,
Novofine® Plus or NovoTwist® disposable needles.
Must be refrigerated until first use.
Store at 36°F – 46°F (2°C – 8°C).
Do not freeze.
Protect from light.
Rx Only
Dispense in this sealed carton.
ATTENTION: Dispense the enclosed Medication Guide to each patient.