NDC Code(s) : 0169-4136-11, 0169-4136-02, 0169-4132-90, 0169-4132-97, 0169-4132-11, 0169-4132-12, 0169-4130-01, 0169-4130-13, 0169-4772-11, 0169-4772-12, 0169-4772-90, 0169-4772-97, 0169-4181-03, 0169-4181-13, 0169-4181-90, 0169-4181-97
Packager : Novo Nordisk
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Ozempicsemaglutide INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Ozempicsemaglutide INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Ozempicsemaglutide INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Ozempicsemaglutide INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Ozempicsemaglutide INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Novo Nordisk(622920320) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Novo Nordisk A/S | 305914798 | MANUFACTURE(0169-4136, 0169-4132, 0169-4130, 0169-4772, 0169-4181) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Novo Nordisk Pharmaceutical Industries, LP | 622920320 | MANUFACTURE(0169-4136, 0169-4132, 0169-4130, 0169-4772, 0169-4181) | |
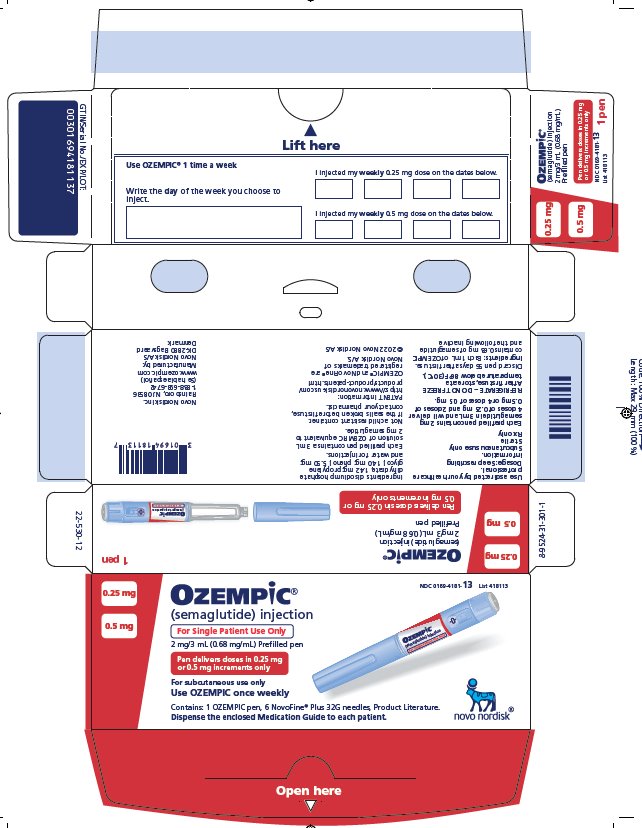
PRINCIPAL DISPLAY PANEL
0.25 mg
0.5 mg
NDC 0169-4181-13List 418113
OZEMPIC®
(semaglutide) injection
For Single Patient Use Only
2 mg/3 mL (0.68 mg/mL) Prefilled pen
Pen delivers doses in 0.25 mg or 0.5 mg increments only
For subcutaneous use only
Use OZEMPIC once weekly
Contains: 1 OZEMPIC pen, 6 NovoFine ® Plus 32G needles, Product Literature.
Dispense the enclosed Medication Guide to each patient.
PRINCIPAL DISPLAY PANEL
1 mg
NDC 0169-4130-13List 413013
OZEMPIC®
(semaglutide) injection
For Single Patient Use Only
4 mg/3 mL (1.34 mg/mL) Prefilled pen
Pen delivers doses in 1 mg increments only
For subcutaneous use only
Use OZEMPIC once weekly
Contains: 1 OZEMPIC pen, 4 NovoFine ® Plus 32G needles, Product Literature.
Dispense the enclosed Medication Guide to each patient.

PRINCIPAL DISPLAY PANEL
2 mg
NDC 0169-4772-12List 477212
OZEMPIC®
(semaglutide) injection
For Single Patient Use Only
8 mg/3 mL (2.68 mg/mL) Prefilled pen
Pen delivers 4 doses of 2 mg only
For subcutaneous use only
Use OZEMPIC once weekly
Contains: 1 OZEMPIC pen, 4 NovoFine ® Plus 32G needles, Product Literature.
Dispense the enclosed Medication Guide to each patient.

PRINCIPAL DISPLAY PANEL
0.25 mg
0.5 mg
NDC 0169-4132-12List 413212
OZEMPIC®
(semaglutide) injection
For Single Patient Use Only
2 mg/1.5 mL (1.34 mg/mL) Prefilled pen
Pen delivers doses in 0.25 mg or 0.5 mg increments only
For subcutaneous use only
Use OZEMPIC once weekly
Contains: 1 OZEMPIC pen, 6 NovoFine ® Plus 32G needles, Product Literature.
Dispense the enclosed Medication Guide to each patient.

PRINCIPAL DISPLAY PANEL
1 mg
NDC 0169-4136-02List 413602
OZEMPIC®
(semaglutide) injection
For Single Patient Use Only
2 mg/1.5 mL (1.34 mg/mL) Prefilled pen
Pen delivers doses in 1 mg increments only
For subcutaneous use only
Use OZEMPIC once weekly
Contains: 2 OZEMPIC pens, 4 NovoFine ® Plus 32G needles, Product Literature.
Dispense the enclosed Medication Guide to each patient.










