NDC Code(s) : 0169-5174-02, 0169-5174-01, 0169-5175-10, 0169-5175-11
Packager : Novo Nordisk
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Activellaestradiol and norethindrone acetate TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Activellaestradiol and norethindrone acetate TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
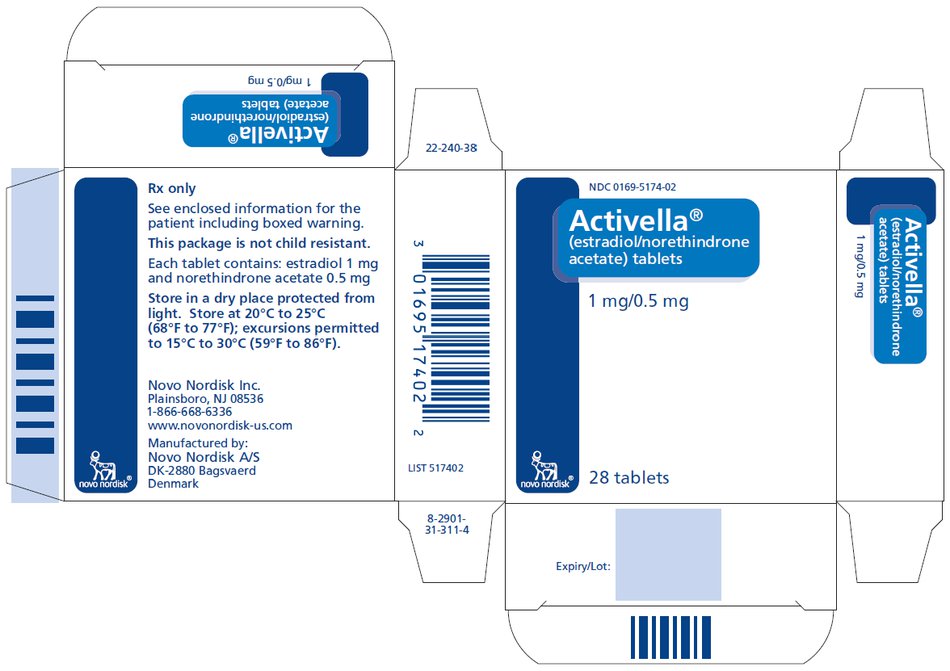
PRINCIPAL DISPLAY PANEL
NDC 0169-5174-02
Activella®
(estradiol/norethindrone
acetate) tablets
1 mg/0.5 mg
28 tablets
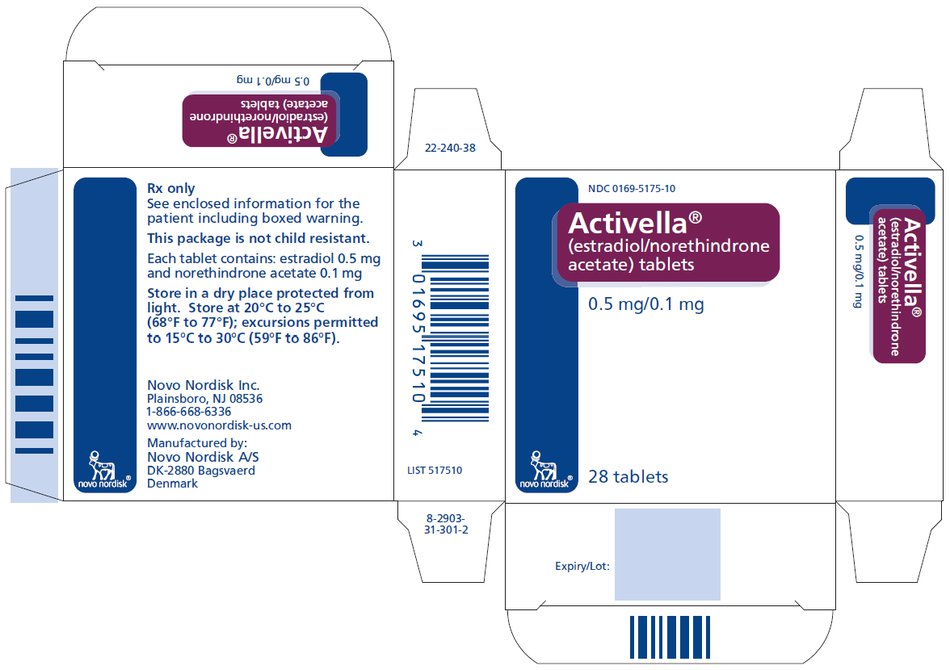
PRINCIPAL DISPLAY PANEL
NDC 0169-5175-10
Activella®
(estradiol/norethindrone
acetate) tablets
0.5 mg/0.1 mg
28 tablets
PRINCIPAL DISPLAY PANEL
NDC 0169-5174-01
List 517401
(Contains: 5 of NDC 0169-5174-02)
Activella®
(estradiol/norethindroneacetate) tablets
Rx only
1 mg/0.5 mg
5-28 packs
See enclosed information for the patient
including boxed warning.
This package is not child resistant.
Store in a dry place protected from light.
Store at 20°C to 25°C (68°F to 77°F); excursions
permitted to 15°C to 30°C (59°F to 86°F).
Novo Nordisk Inc.
Plainsboro, NJ 08536
1-866-668-6336
www.novonordisk-us.com
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd
Denmark

PRINCIPAL DISPLAY PANEL
NDC 0169-5175-11
LIST 517511
(Contains: 5 of NDC 0169-5175-10)
Activella®
(estradiol/norethindroneacetate) tablets
Rx only
0.5 mg/0.1 mg
5-28 packs
See enclosed information for the patient including
boxed warning.
This package is not child resistant.
Store in a dry place protected from light. Store at
20°C to 25°C (68°F to 77°F); excursions permitted to
15°C to 30°C (59°F to 86°F).
Novo Nordisk Inc.
Plainsboro, NJ 08536
1-866-668-6336
www.novonordisk-us.com
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd
Denmark










