NDC Code(s) : 0169-7825-01, 0169-7850-01, 0169-7810-01, 0169-7815-01, 0169-7820-01, 0169-7830-01
Packager : Novo Nordisk
Category : PLASMA DERIVATIVE
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| NOVOEIGHT(antihemophilic factor, recombinant) KIT | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| NOVOEIGHT(antihemophilic factor, recombinant) KIT | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| NOVOEIGHT(antihemophilic factor, recombinant) KIT | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| NOVOEIGHT(antihemophilic factor, recombinant) KIT | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| NOVOEIGHT(antihemophilic factor, recombinant) KIT | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| NOVOEIGHT(antihemophilic factor, recombinant) KIT | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LABELER - Novo Nordisk(622920320) |
PRINCIPAL DISPLAY PANEL
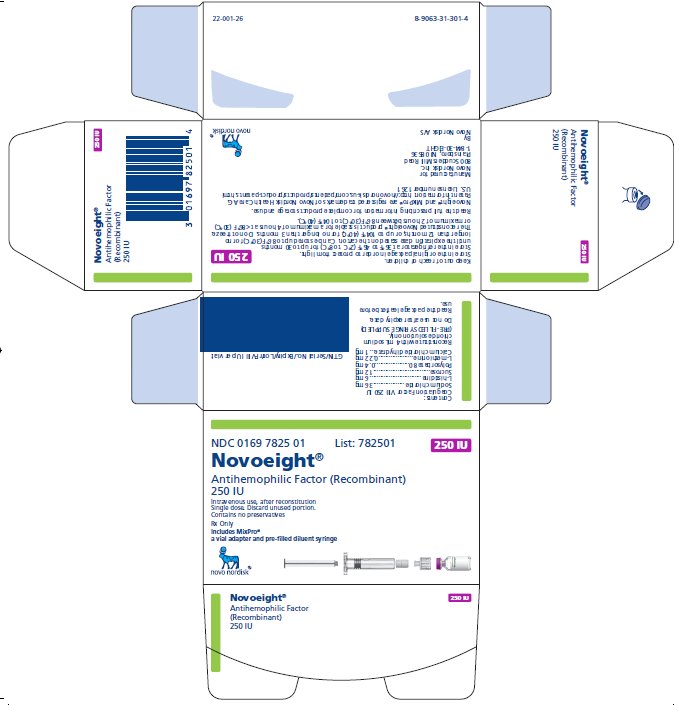
250 IU Carton
NDC 0169 7825 01 List: 782501
Novoeight®
Antihemophilic Factor (Recombinant)
250 IU
Intravenous use, after reconstitution
Single dose. Discard unused portion.
Contains no preservatives
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe
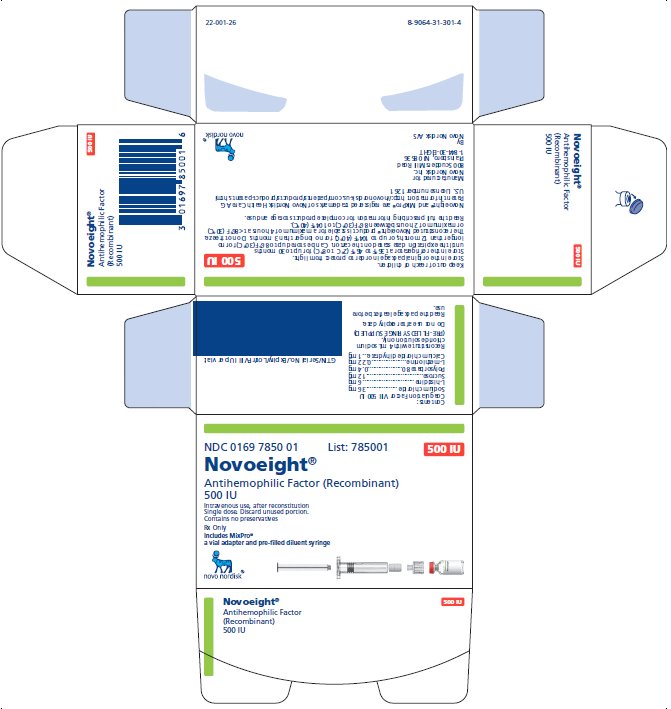
PRINCIPAL DISPLAY PANEL
NDC 0169 7850 01 List: 785001
Novoeight®
Antihemophilic Factor (Recombinant)
500 IU
Intravenous use, after reconstitution
Single dose. Discard unused portion.
Contains no preservatives
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe
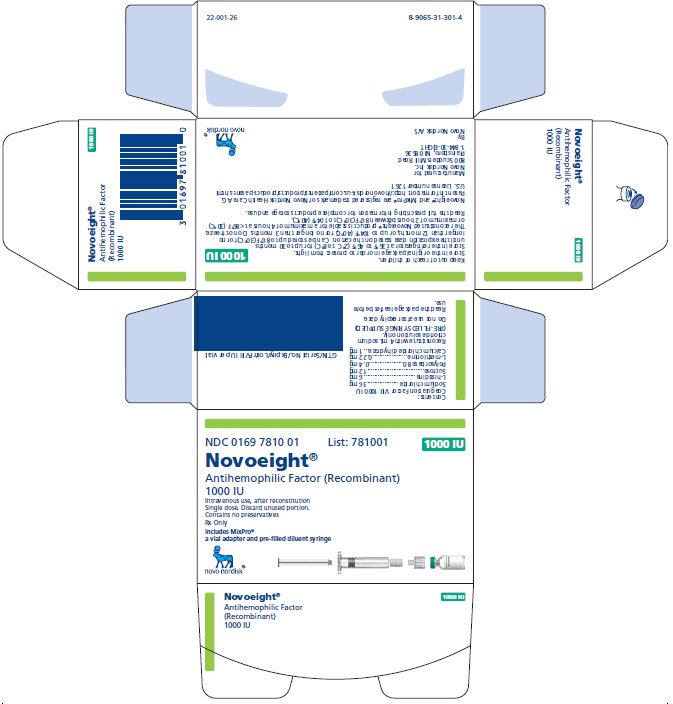
PRINCIPAL DISPLAY PANEL
NDC 0169 7810 01 List: 781001
Novoeight®
Antihemophilic Factor (Recombinant)
1000 IU
Intravenous use, after reconstitution
Single dose. Discard unused portion.
Contains no preservatives
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe
PRINCIPAL DISPLAY PANEL
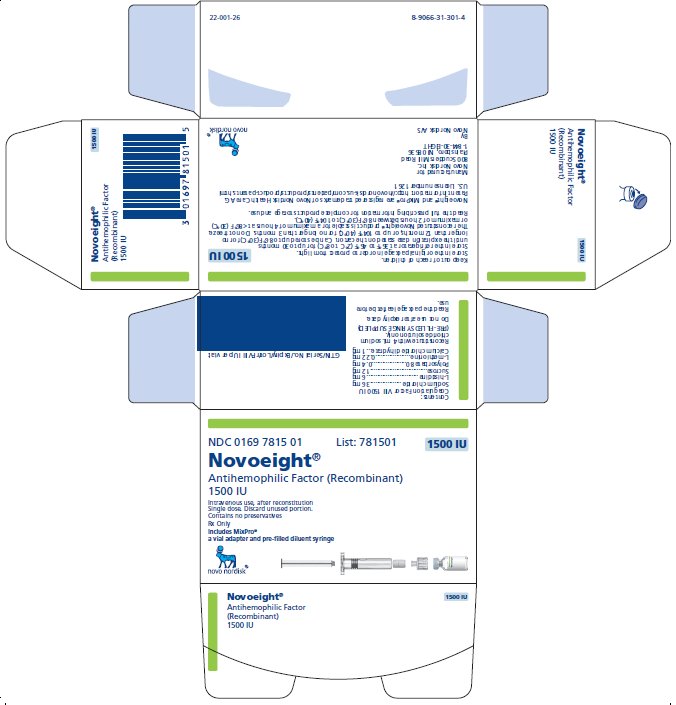
NDC 0169 7815 01 List: 781501
Novoeight®
Antihemophilic Factor (Recombinant)
1500 IU
Intravenous use, after reconstitution
Single dose. Discard unused portion.
Contains no preservatives
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe
PRINCIPAL DISPLAY PANEL
NDC 0169 7820 01 List: 782001
Novoeight®
Antihemophilic Factor (Recombinant)
2000 IU
Intravenous use, after reconstitution
Single dose. Discard unused portion.
Contains no preservatives
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe

PRINCIPAL DISPLAY PANEL
NDC 0169 7830 01 List: 783001
Novoeight®
Antihemophilic Factor (Recombinant)
3000 IU
Intravenous use, after reconstitution
Single dose. Discard unused portion.
Contains no preservatives
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe

PRINCIPAL DISPLAY PANEL
NDC 0169 7829 11
Novoeight®
Antihemophilic Factor
(Recombinant)
Store in a refrigerator 36°F -
46°F (2-8°C)

PRINCIPAL DISPLAY PANEL
NDC 0169 7851 11
Novoeight®
Antihemophilic Factor
(Recombinant)
Store in a refrigerator 36°F -
46°F (2-8°C)

PRINCIPAL DISPLAY PANEL
NDC 0169 7811 11
Novoeight®
Antihemophilic Factor
(Recombinant)
Store in a refrigerator 36°F -
46°F (2-8°C)

PRINCIPAL DISPLAY PANEL
NDC 0169 7855 11
Novoeight®
Antihemophilic Factor
(Recombinant)
Store in a refrigerator 36°F -
46°F (2-8°C)

PRINCIPAL DISPLAY PANEL
NDC 0169 7821 11
Novoeight®
Antihemophilic Factor
(Recombinant)
Store in a refrigerator 36°F -
46°F (2-8°C)

PRINCIPAL DISPLAY PANEL
NDC 0169 7831 11
Novoeight®
Antihemophilic Factor
(Recombinant)
Store in a refrigerator 36°F -
46°F (2-8°C)

PRINCIPAL DISPLAY PANEL
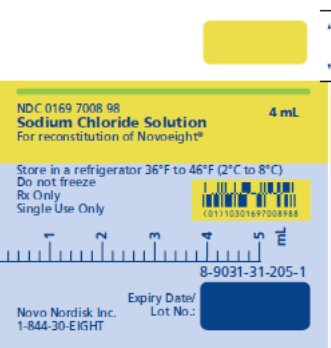
NDC 0169 7008 98
Sodium Chloride Solution
For reconstitution of Novoeight®
Store in a refrigerator 36°F to 46°F (2°C to 8°C)
Do not freeze
Rx Only
Single Use Only