NDC Code(s) : 0178-0891-30
Packager : Mission Pharmacal Company
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CitraNatal Assureascorbic acid, calcium citrate, iron, vitamin d, dl- alpha- tocopherol acetate, thiamine, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, iodine, zinc, copper, docusate sodium, doconexent and icosapent KIT | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| LABELER - Mission Pharmacal Company(008117095) |
| REGISTRANT - Mission Pharmacal Company(927726893) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Mission Pharmacal Company | 927726893 | manufacture(0178-0891) | |
PRINCIPAL DISPLAY PANEL
CitraNatal Assure Retail Blister Pack
NDC 0178–0891–30

PRINCIPAL DISPLAY PANEL
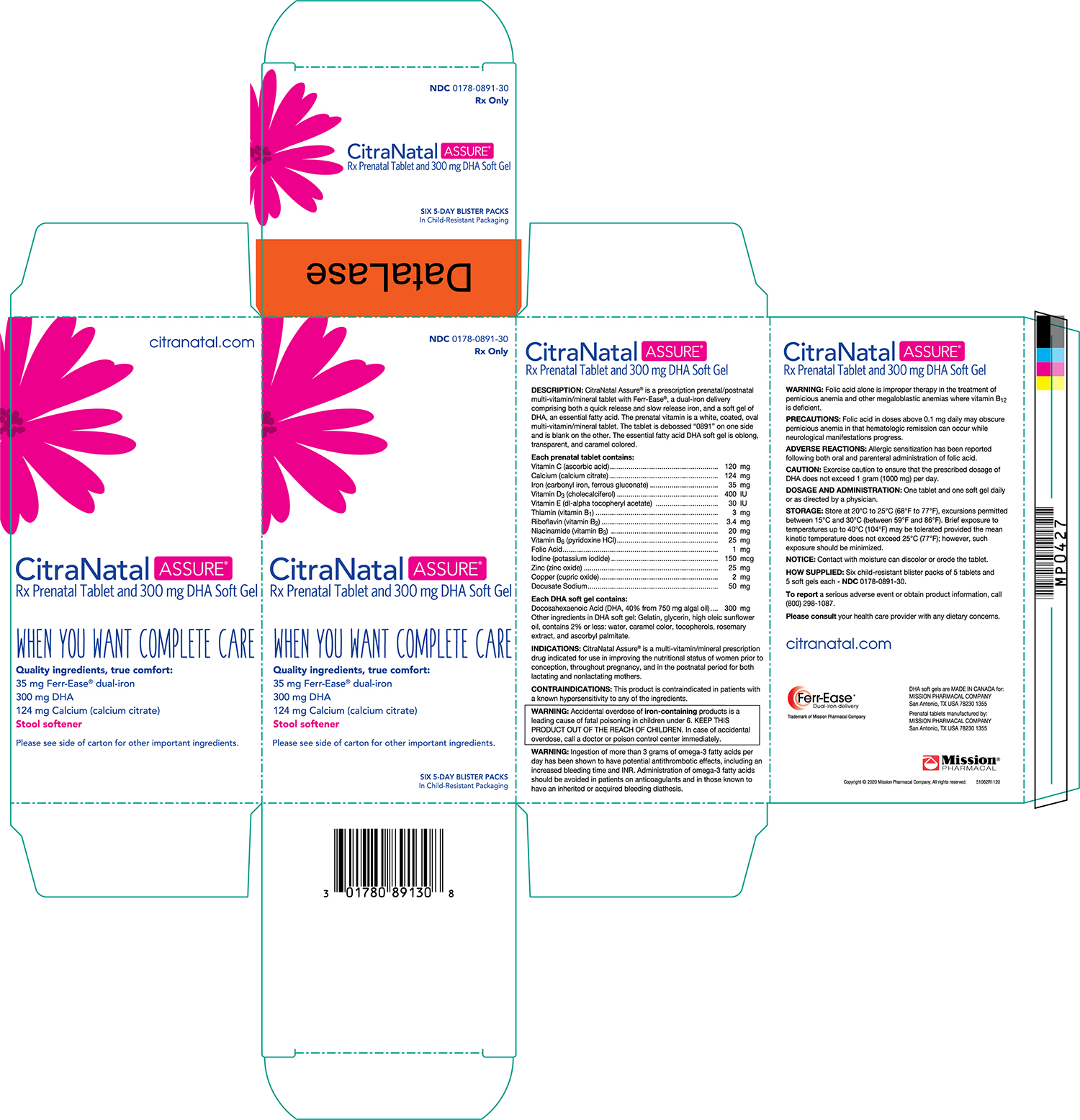 Retail Carton
Retail Carton









