NDC Code(s) : 0206-3501-01, 0206-3501-10, 0206-2501-01, 0206-2501-10, 0206-4501-01, 0206-4501-10, 0206-5501-01
Packager : Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Zosyntazobactam sodium and piperacillin sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Zosyntazobactam sodium and piperacillin sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Zosyntazobactam sodium and piperacillin sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Zosyn tazobactam sodium and piperacillin sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 0206-3501-01
3.375 gram Single Use Vial
Zosyn ®
(piperacillin and
tazobactam for
injection, USP)
3.375 gram
For IV Use Only
Rx only
PREMIERPro™ Rx

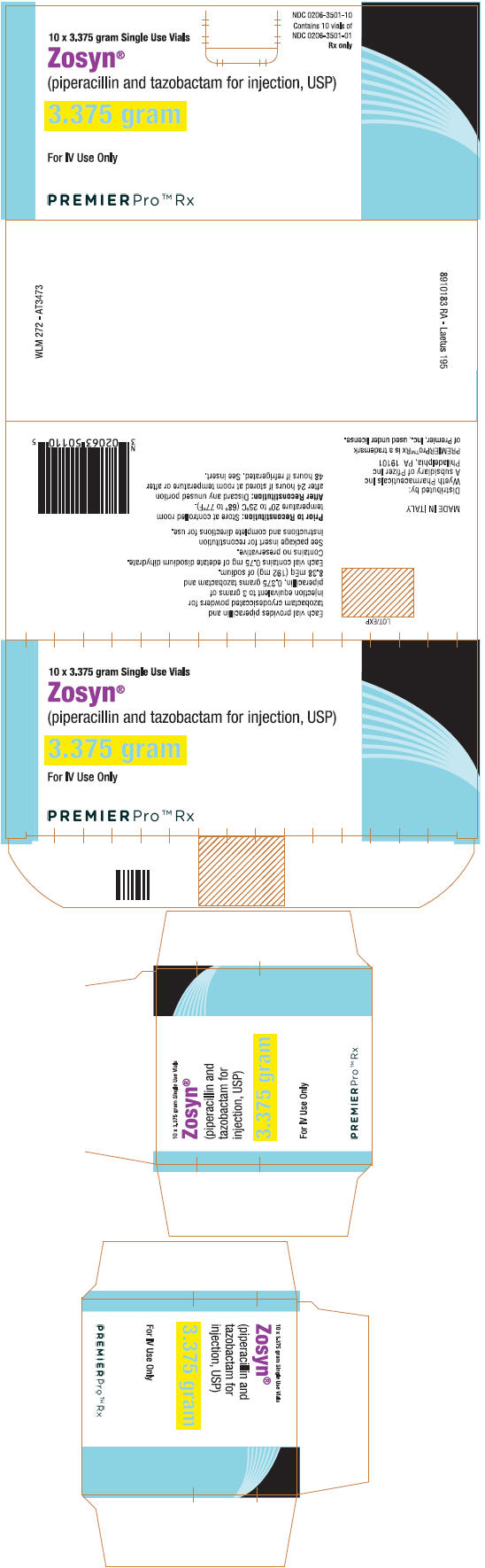
PRINCIPAL DISPLAY PANEL
NDC 0206-3501-10
Contains 10 vials of
NDC 0206-3501-01
Rx only
10 x 3.375 gram Single Use Vials
Zosyn®
(piperacillin and tazobactam for injection, USP)
3.375 gram
For IV Use Only
PREMIERPro™ Rx
PRINCIPAL DISPLAY PANEL
NDC 0206-2501-01
2.25 gram Single Use Vial
Zosyn®
(piperacillin and
tazobactam for
injection, USP)
2.25 gram
For IV Use Only
Rx only
PREMIERPro™ Rx

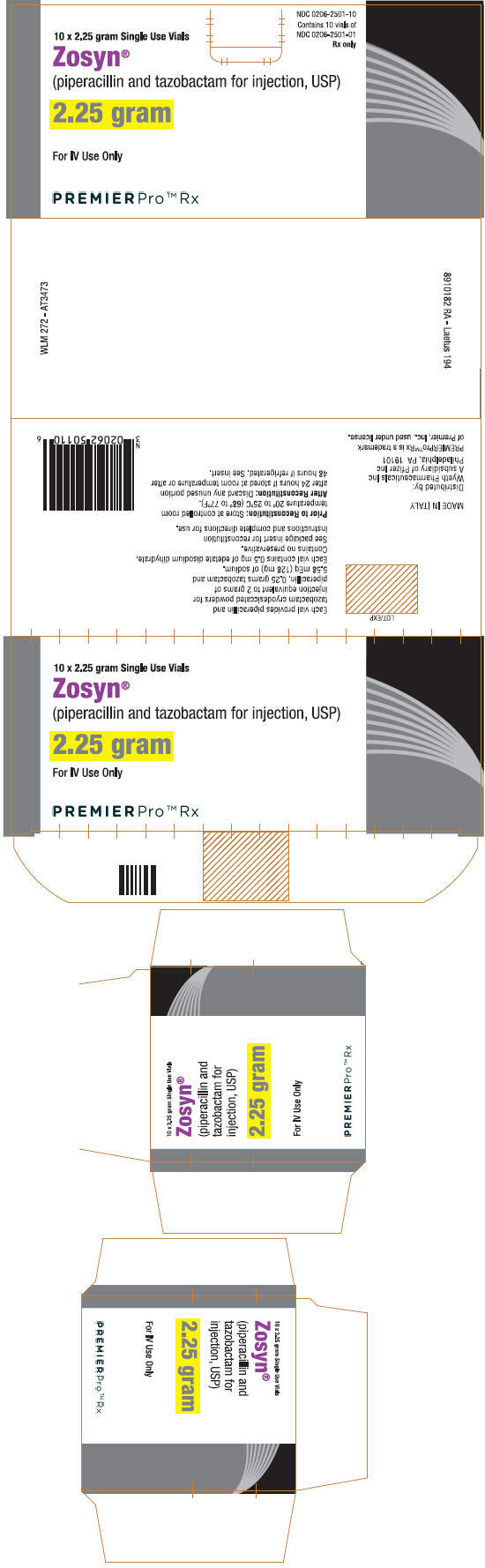
PRINCIPAL DISPLAY PANEL
NDC 0206-2501-10
Contains 10 vials of
NDC 0206-2501-01
Rx only
10 x 2.25 gram Single Use Vials
Zosyn®
(piperacillin and tazobactam for injection, USP)
2.25 gram
For IV Use Only
PREMIERPro™ Rx
PRINCIPAL DISPLAY PANEL
NDC 0206-4501-01
4.5 gram Single Use Vial
Zosyn ®
(piperacillin and
tazobactam for
injection, USP)
4.5 gram
For IV Use Only
Rx only
PREMIERPro™ Rx

PRINCIPAL DISPLAY PANEL
NDC 0206-4501-10
Contains 10 of
NDC 0206-4501-01
Rx only
10 x 4.5 gram Single Use Vials
Zosyn®
(piperacillin and tazobactam for Injection, USP)
4.5 gram
For IV Use Only
PREMIERPro™ Rx

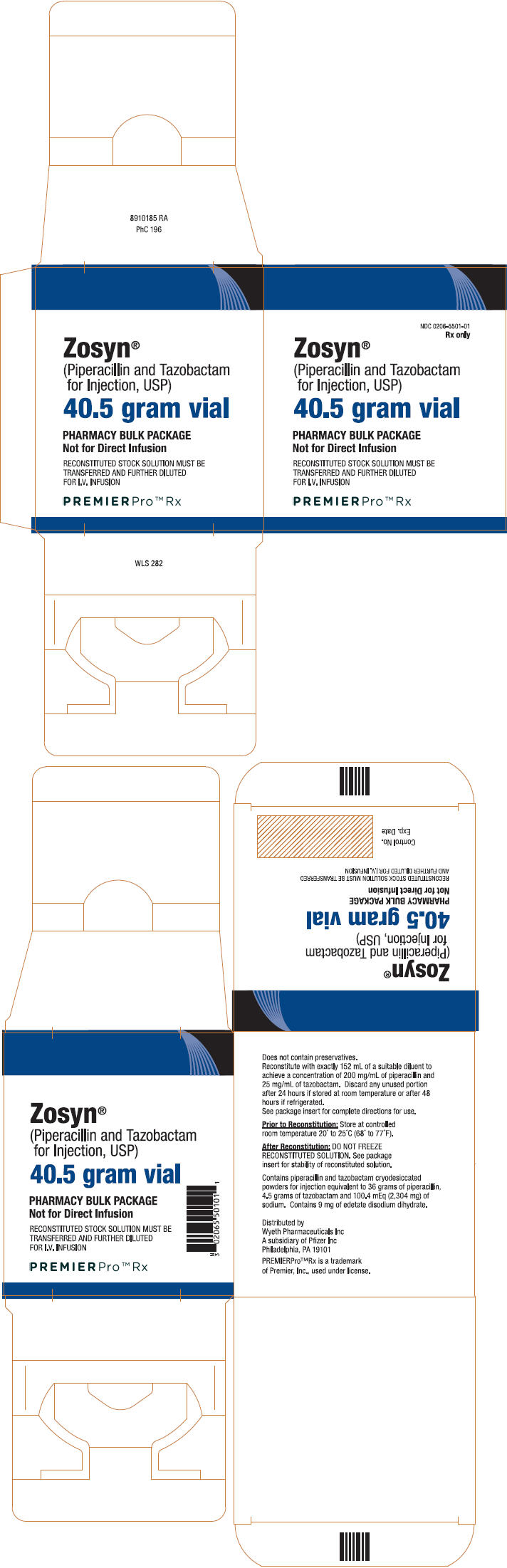
PRINCIPAL DISPLAY PANEL
PREMIERPro™ Rx
NDC 0206-5501-01
Zosyn
®
(Piperacillin and Tazobactam
for Injection, USP)
Rx only
40.5 gram vial
PHARMACY BULK PACKAGE
Not for Direct Infusion
Distributed by Wyeth Pharmaceuticals Inc
A subsidiary of Pfizer Inc, Philadelphia, PA 19101
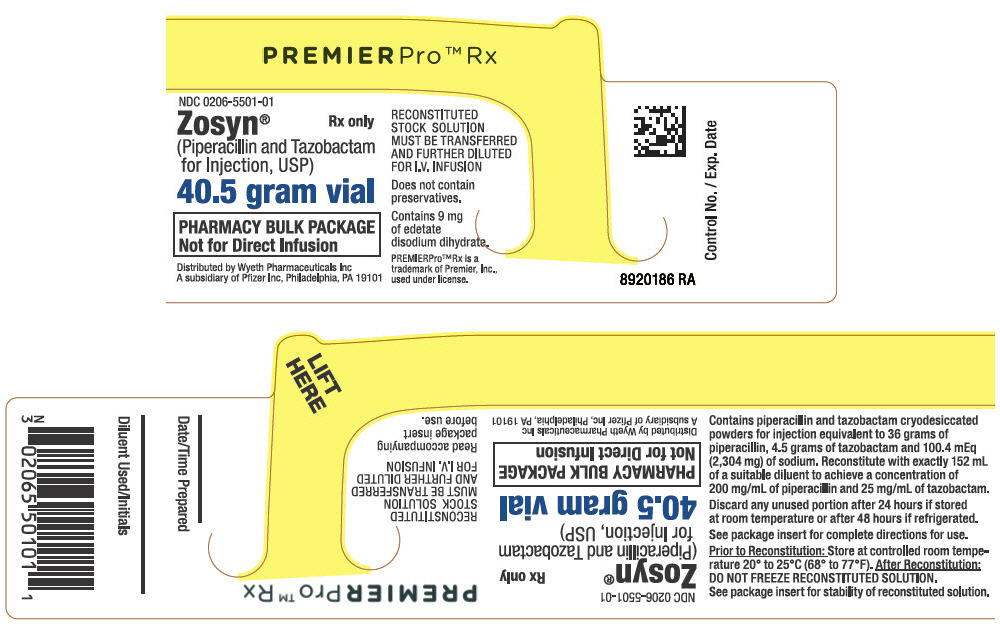
PRINCIPAL DISPLAY PANEL
NDC 0206-5501-01
Rx only
Zosyn
®
(Piperacillin and Tazobactam
for Injection, USP)
40.5 gram vial
PHARMACY BULK PACKAGE
Not for Direct Infusion
RECONSTITUTED STOCK SOLUTION MUST BE
TRANSFERRED AND FURTHER DILUTED
FOR I.V. INFUSION
PREMIERPro™ Rx