NDC Code(s) : 0245-0169-01, 0245-0169-20, 0245-0168-06, 0245-0168-12, 0245-0168-20
Packager : Upsher-Smith Laboratories, Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cleniasulfacetamide sodium and sulfur CREAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Cleniasulfacetamide sodium and sulfur CREAM | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 0245-0169-01
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Emollient Cream
1 oz (28 g)
UPSHER-SMITH

PRINCIPAL DISPLAY PANEL
NDC 0245-0169-01
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Emollient Cream
1 oz (28 g)
UPSHER-SMITH

PRINCIPAL DISPLAY PANEL
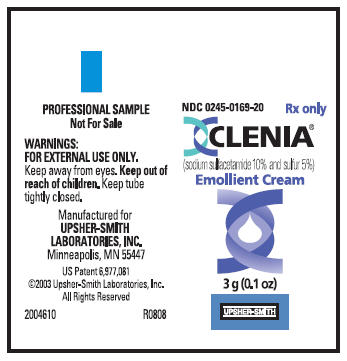
NDC 0245-0169-20
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Emollient Cream
3 g (0.1 oz)
UPSHER-SMITH
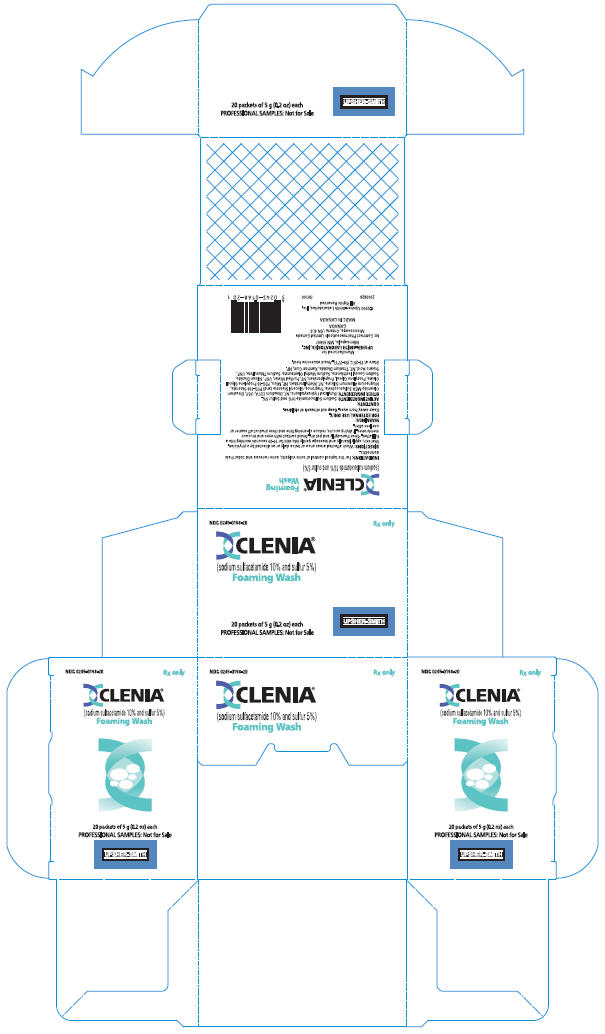
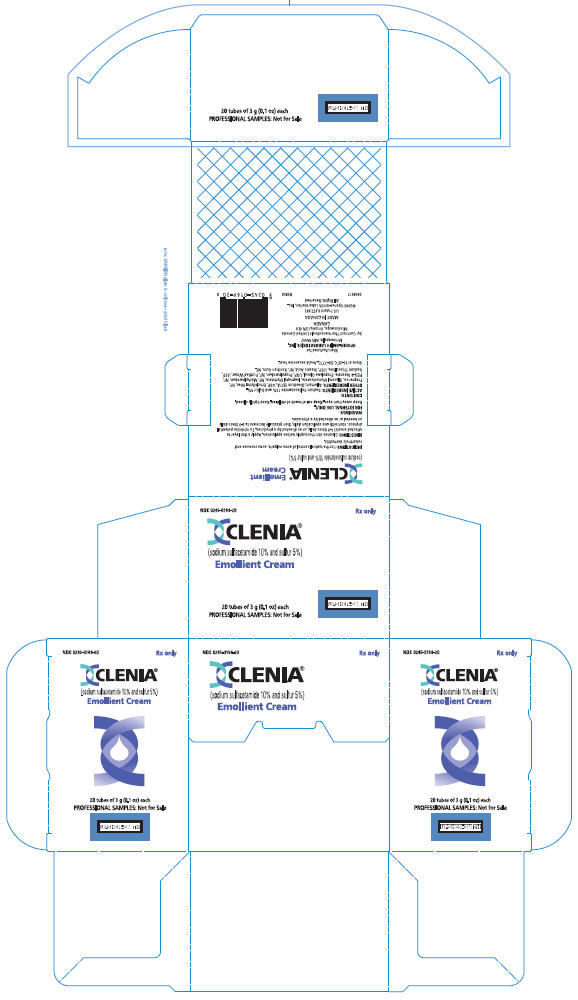
PRINCIPAL DISPLAY PANEL
NDC 0245-0169-20
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Emollient Cream
20 tubes of 3 g (0.1 oz) each
PROFESSIONAL SAMPLES: Not for Sale
UPSHER-SMITH
PRINCIPAL DISPLAY PANEL
NDC 0245-0168-06
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
6 oz (170 g)
UPSHER-SMITH
2003765
R0505


PRINCIPAL DISPLAY PANEL
NDC 0245-0168-06
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
6 oz (170 g)
UPSHER-SMITH

PRINCIPAL DISPLAY PANEL
NDC 0245-0168-12
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
12 oz (340 g)
UPSHER-SMITH
2003771
R0505


PRINCIPAL DISPLAY PANEL
NDC 0245-0168-12
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
12 oz (340 g)
UPSHER-SMITH

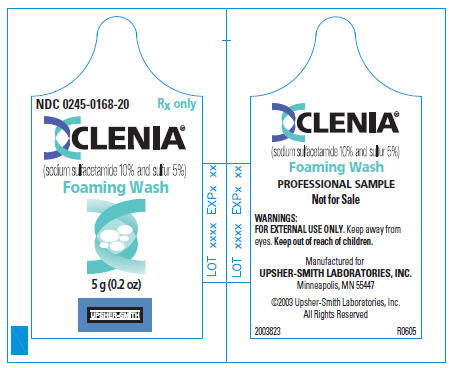
PRINCIPAL DISPLAY PANEL
NDC 0245-0168-20
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
5 g (0.2 oz)
UPSHER-SMITH
PRINCIPAL DISPLAY PANEL
NDC 0245-0168-20
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
20 packets of 5 g (0.2 oz) each
PROFESSIONAL SAMPLES: Not for Sale
UPSHER-SMITH