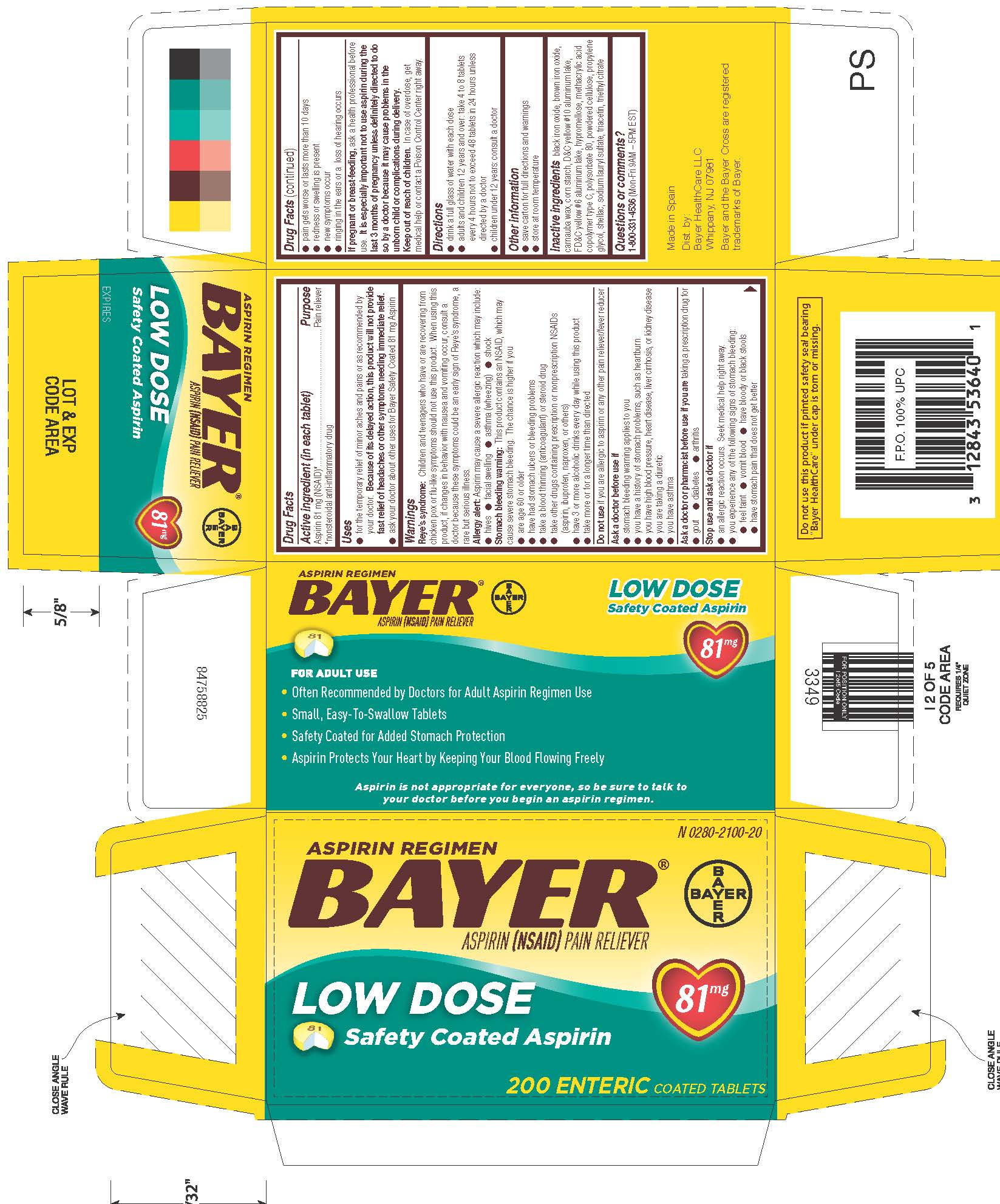
NDC Code(s) : 0280-2100-32, 0280-2100-12, 0280-2100-20, 0280-2100-30, 0280-2100-40
Packager : Bayer HealthCare LLC.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Bayer Aspirin TABLET | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Bayer HealthCare LLC.(112117283) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Bayer HealthCare LLC Consumer Care | 072827066 | manufacture(0280-2100), pack(0280-2100) | |
PRINCIPAL DISPLAY PANEL
N 0280-2100-32
ASPIRIN REGIMEN
BAYER
®
ASPIRIN
(NSAID) PAIN RELIEVER
LOW DOSE
81mg
Safety Coated
Aspirin
32 ENTERIC COATED TABLETS