NDC Code(s) : 0295-1224-02
Packager : Denison Pharmaceuticals, LLC.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DEBROXCarbamide Peroxide LIQUID | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
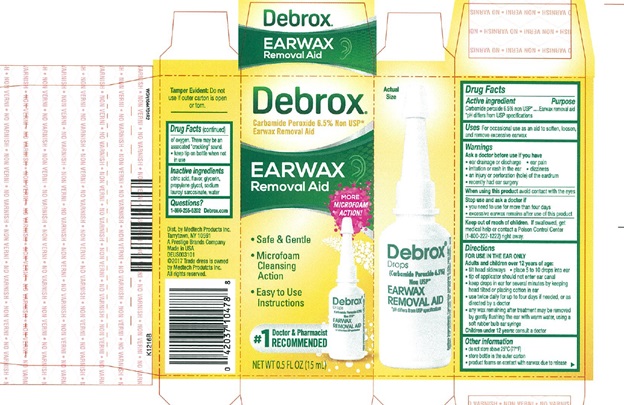
PRINCIPAL DISPLAY PANEL
Debrox® DROPS
Carbamide Peroxide 6.5% Non USP*
Earwax Removal Aid
½ FL OZ (15mL)